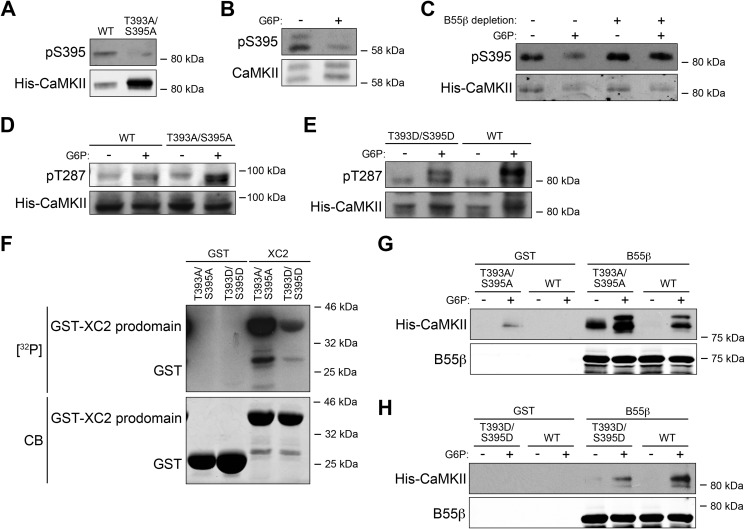
FIGURE 5.
B55β-mediated Thr393/Ser395 dephosphorylation contributes to CaMKII activation. A, Xenopus WT and T393A/S395A mutant CaMKIIγ expressed from baculoviral vectors in SF9 cells were analyzed by Ser(P)395 immunoblotting. B, CaM-Sepharose was incubated with Xenopus egg extracts treated with or without G6P, retrieved by centrifugation, and analyzed by Ser(P)395 immunoblotting. C, B55β-depleted or undepleted Xenopus egg extracts with the addition of recombinant CaMKIIγ were treated with or without G6P. Recombinant CaMKIIγ was precipitated with CaM-Sepharose. The beads were retrieved by centrifugation and analyzed for Ser(P)395. D, CaMKIIγ WT or T393A/S395A mutant was added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P and analyzed for pT287. E, CaMKIIγ WT or T393D/S395D mutant was treated and processed as in D. F, GST tagged Xenopus caspase-2 prodomain or GST bound to glutathione-Sepharose was incubated with Xenopus CaMKIIγ T393A/S395A or T393D/S395D mutants, calmodulin, and [γ-32P] ATP at 30 °C for 0.5 h. Samples were resolved by SDS-PAGE and detected by autoradiography. CB, Coomassie Blue. G, Xenopus CaMKIIγ WT or T393A/S395A mutant proteins were added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P for 0.5 h at room temperature and incubated with GST-B55β or GST bound to glutathione-Sepharose for 1 h at 4 °C. The beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting. H, Xenopus CaMKIIγ WT or T393D/S395D mutant proteins were added into Xenopus egg extracts for 0.5 h at room temperature, and then the extracts were treated with or without G6P for 0.5 h at room temperature and incubated with GST-B55β or GST bound to glutathione-Sepharose for 1 h at 4 °C. Beads were retrieved by centrifugation and analyzed for CaMKII or B55β immunoblotting.