Background: Periodical ecdysis occurs in insects with molting fluids accumulated among the old and new cuticles.
Results: Molting fluid is a mixture containing many unknown proteins to inhibit microbian infection and regulate ecdysis.
Conclusion: Insects produce molting fluids for protecting delicate insects and guaranteeing successful ecdysis.
Significance: Molting proteins may be targets useful for pesticide development in the future.
Keywords: Bacteria, Epidermis, Fungi, Gene Silencing, Innate Immunity, Insect, Proteomics, Shedding
Abstract
Molting fluid accumulates between the old and new cuticles during periodical ecdysis in Ecdysozoa. Natural defects in insect ecdysis are frequently associated with melanization (an immunity response) occurring primarily in molting fluids, suggesting that molting fluid may impact immunity as well as affect ecdysis. To address this hypothesis, proteomic analysis of molting fluids from Bombyx mori during three different types of ecdysis was performed. Many proteins were newly identified, including immunity-related proteins, in each molting fluid. Molting fluids inhibited the growth of bacteria in vitro. The entomopathogenic fungi Beauveria bassiana, which can escape immune responses in feeding larvae, is quickly recognized by larvae during ecdysis, followed by melanization in molting fluid and old cuticle. Fungal conidia germination was delayed, and no hyphae were detected in the hemocoels of pharate instar insects. Molting fluids protect the delicate pharate instar insects with extremely thin cuticles against microorganisms. To explore the function of molting fluids in ecdysis regulation, based on protein similarity, 32 genes were selected for analysis in ecdysis regulation through RNAi in Tribolium castaneum, a model commonly used to study integument development because RNAi is difficult to achieve in B. mori. We identified 24 molting proteins that affected ecdysis after knockdown, with different physiological functions, including old cuticle protein recycling, molting fluid pressure balance, detoxification, and signal detection and transfer of molting fluids. We report that insects secrete molting fluid for protection and regulation of ecdysis, which indicates a way to develop new pesticides through interrupting insect ecdysis in the future.
Introduction
Ecdysis occurs in Ecdysozoa, which includes arthropods (e.g. insects, spiders, and crustaceans) and nematodes, with more than 4.5 million living species (1). Insects undergo a process of development different from that of mammals. After hatching from eggs, insects experience several different developmental stages during the life cycle: larva, pupa, and adult (complete metamorphosis) and larva (nymph) and adult (incomplete metamorphosis) (2). When entering a new developmental stage, insects must shed the old cuticle.
The insect integument is a layer of epidermal cells covered by acellular and solid cuticle to prevent excess water evaporation as well as pathogen invasion (3). The insect cuticle is divided into two layers: the outermost epicuticle, which contains mainly water-resistant wax, and the procuticle, located between the epicuticle and epidermis. The procuticle has numerous layers of interwoven fibrous chitin and proteins (3). Before ecdysis or eclosion, new epicuticle and procuticle are deposited onto epidermal cells. For arthropods, it takes time for the tanning of new cuticle to occur after ecdysis. After ecdysis, pathogens like fungi can easily penetrate new tender skin. It remains unclear how the newly molted arthropods defend themselves during this period. Molting fluid probably produced by epidermal cells and dermal glands fills the space between the old and new cuticles during ecdysis (3). Chitinase and β-N-acetylglucosaminidase were identified in molting fluids, with chitinases binding to the old cuticle to degrade chitin (4). Two molting fluid peptidases (MFP-1 and MFP-2) purified from Manduca sexta were concluded to degrade cuticle proteins into polypeptide fragments and free amino acids for recycling (3). Although it was concluded that there are probably additional proteins in the molting fluids, no proteins other than those listed above were identified and characterized. Initially, we observed that the unsuccessful insect ecdysis induced by pathogens is associated with melanization, an innate immunity response, mainly in the molting fluids of the silkworm, Bombyx mori. This suggests that molting fluid has functions of immunity protection and ecdysis regulation. To understand integument immunity in arthropods undergoing different types of ecdysis as well as the factors regulate ecdysis, it is important to identify all proteins present in molting fluids.
Here we first performed a proteomic analysis of insect molting fluids using the silkworm B. mori as a model. We identified 109, 97, and 77 proteins in the silkworm larval-larval, larval-pupal, and pupal-adult molting fluids, respectively. Insect molting fluid is a mixture containing various proteins that have a close relationship with physiological functions, such as recycling of old cuticle proteins and chitin, balance of molting fluid pressure, detoxification of toxins, and signal detection and transduction. Molting immunity-related proteins coordinately defend against bacteria and fungi in different ways. It has been demonstrated that chitinolytic enzymes found in silkworm molting fluids can affect Tribolium castaneum ecdysis (5–7), which indicates that both species probably share similar molting proteins. Because RNAi has generally not been successful in the silkworm (8), we used the RNAi sensitive Tribolium as a model to identify molting proteins involved in detoxification, recycling, and signal transduction that affects ecdysis and development following the knockdown of corresponding genes. Therefore, it appears that insects secrete molting fluid for immune protection of pharate instar insect bodies and regulation of ecdysis.
EXPERIMENTAL PROCEDURES
Insect Feeding, 20-Hydroxyecdysone Injection, Dissection, and Tissue Section
Silkworm larvae (Nistari) were reared on mulberry leaves at 25 °C under a 12-h photoperiod. Silkworm larvae at 12 h on day 0 of the fifth larval stage (V-0; 12 h) were injected with 20 μg of 20-hydroxyecdysone (20-E)2 (Sigma) or dimethyl sulfoxide (DMSO) to induce a molting-like stage with new cuticle produced. After bleeding, abdominal integuments were sampled from larvae at different developmental stages and washed in autoclaved 0.85% NaCl solution. The attached fat body, muscle, and spiracles were removed. Integuments were dissected on day 3 of the fourth larval stage (IV-3) as well as at different times (6, 12, and 18 h) during the fourth molting stage (IV-M), day 0 and 2 of the fifth larval stage (V-0 and V-2), 0 h postpupation (P-0), day 6 of pupa (P-6), and 12, 24, and 36 h post-20-E injection for proteins or tissue sections as required. Plasma sampled from naive larvae on V-2 was used as a control if necessary. Protein concentrations were determined as described (9). Tissue section was performed as described (10). Slides were stained using a mixture of hematoxylin and eosin solution to show morphological changes or using Calcofluor White stain (Sigma) to detect insect chitin (11). All pictures were taken under a fluorescent microscope (Olympus BX51) using differential interference contrast with the appropriate filter.
Molting Fluid Collection
To collect the molting fluid from the fourth larval molting stage, the old cuticle was quickly removed using clean tweezers and dipped in 200 μl of molting fluid collection buffer (10 mm Tris, 100 mm NaCl, 5 mm PMSF, 5 mm EDTA, pH 8.0) containing 20 μl of saturated phenylthiourea solution when head capsule slippage occurred. The old cuticle was squeezed against the tube to remove solution after being placed on ice for 10 s and then discarded. The solution was centrifuged at 12,000 × g at 4 °C for 5 min. The supernatant (larval-larval molting fluid) was transferred to a new tube and stored at −80 °C for future use. Molting fluids before pupation or eclosion were collected and transferred into molting fluid collection buffer, as described previously (12). Phenylthiourea was not added if phenoloxidase (PO) activity was assayed.
Gel Digestion, LC-MS/MS, and Bioinformatics Analysis
Molting fluids (20 μg) were separated on 12% SDS-polyacrylamide gels. Each gel lane was cut into several bands for LC-MS/MS assays. Briefly, gel pieces were destained using 30% acetonitrile prepared in 100 mm NH4HCO3 and dried in a vacuum centrifuge. The in-gel proteins were reduced in dithiothreitol buffer (10 mm DTT dissolved in 100 mm NH4HCO3) for 30 min at 56 °C and then alkylated using iodoacetamide (200 mm IAA prepared in 100 mm NH4HCO3) in the dark at room temperature for 30 min. After washing in 100 mm NH4HCO3 and acetonitrile, respectively, gel pieces were digested overnight in 12.5 ng/μl trypsin solution (prepared in 25 mm NH4HCO3). The peptides were extracted three times using a solution containing 60% acetonitrile and 0.1% TFA, and after pooling, the extracts were dried completely. Then the EttanTM MDLC system (GE Healthcare) was applied for desalting and separation of peptides. Samples were desalted on reversed phase (RP) trap columns (Zorbax 300 SB C18, Agilent Technologies) and then separated on an RP column (150-μm inner diameter, 100-mm length; Column Technology Inc.). Mobile phase A (0.1% formic acid in HPLC-grade water) and the mobile phase B (0.1% formic acid in acetonitrile) were selected. The separation was performed at a flow rate of 2 μl/min using a linear gradient of 4–50% buffer B for 50 min, 50–100% buffer B for 4 min, and 100% buffer B for 6 min. LTQ Velos (Thermo Scientific) equipped with a microspray interface was connected to the LC setup for eluted peptide detection. Data-dependent MS/MS spectra were obtained simultaneously. Each scan cycle consisted of one full scan mass spectrum (m/z 300–1800) followed by 20 MS/MS events of the most intense ions with the following dynamic exclusion settings: repeat count, 2; repeat duration, 30 s; exclusion duration, 90 s. The silkworm protein sequence database was downloaded from NCBI, using the keyword Bombyx mori plus those from the silkworm genome database (13). MS/MS spectra were automatically searched against this database using the BioworksBrowser rev. 3.1 (Thermo Electron, San Jose, CA). Protein identification results were extracted from SEQUEST files with BuildSummary. The peptides were constrained to be tryptic, and up to two missed cleavages were allowed. Carbamidomethylation of cysteines was treated as a fixed modification, whereas oxidations of methionine residues were considered as a variable modification. The mass tolerance allowed for the precursor ions was 2.0 Da, and that for fragment ions was 0.8 Da, respectively. The protein identification criteria were based on Delta CN (≥0.1) and cross-correlation scores (Xcorr; one charge ≥1.9, two charges ≥2.2, three charges ≥3.75). The molecular identity and domains were predicted using the online Pfam program. Gene ontology was analyzed using BGI WEGO. To identify the corresponding proteins in other organisms, the BLASTP program was used to search for the corresponding genes in Homo sapiens, Drosophila melanogaster, and T. castaneum. The most highly related proteins from humans, flies, and T. castaneum were compared with the corresponding protein identified in the silkworm molting fluids using the ClustalW alignment program.
Enzyme Activity Assay
To assay different enzyme activities in integuments, tissues were sonicated in PBS buffer (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 2 mm KH2PO4, pH 7.4) and centrifuged at 12,000 × g at 4 °C for 5 min. The supernatant was used immediately or transferred to a new tube and stored at −80 °C for future use. Approximately 30 μg of cell lysates or 30 μg of molting fluid were loaded on each lane for native gel separation. Methods to detect superoxide dismutase (14), catalase (15), protease (16), and esterase (17) activities in a native gel were performed as described previously.
To detect PO activity in molting fluids, each type of molting fluid (10 μg) was added directly to 100 μl of dopamine solution (10 mm dopamine dissolved in 10 mm Tris buffer, pH 7.5). The blank contained 5 μl of saturated phenylthiourea before adding molting fluids. Absorbance was read at 490 nm using the EXPERT 96 microplate reader (Biochrom, Holliston, MA) as described previously (10). One unit of PO was defined as ΔA490/min = 0.001.
Detection of total chitinase activity was performed as described previously (18). Briefly, each type of molting fluid (100 μg) or chitinase (50 μg; Sigma) was incubated with 15 μl of chitin azure solution (3 μg/μl dissolved in 10 mm Tris buffer, pH 7.5; Sigma) at room temperature for 1 h. The blank contained no chitin azure. The above mixtures were terminated by heating at 100 °C for 5 min and were then brought to room temperature, followed by centrifuging at 13,000 × g for 2 min. The absorbance of the supernatant was read at 560 nm and used to determine chitinase activity as described (18).
Effect of Molting Fluids on the Growth of Microbial Cells
A single colony of Escherichia coli (Gram-negative) or Bacillus subtilis (Gram-positive) was inoculated into 3 ml of Luria-Bertani (LB) medium and grown at 37 °C for 12 h at 200 rpm. Bacteria were centrifuged at 3,000 × g for 5 min, and the supernatants were removed. The pellets were separately suspended in 0.85% autoclaved NaCl solution. The optical density was measured at 600 nm (A600). Molting fluids were filtered to remove possible microorganisms and concentrated by ultrafiltration after changing the buffer. The final concentrations were adjusted to 10 μg/μl. Next, 30 μl of filtered molting fluids or 0.85% NaCl solution (control) were mixed with 5 μl of suspended bacteria (E. coli, 0.0003 A600; B. subtilis, 0.003 A600 after serial dilution), respectively, and the above mixtures were incubated at 25 °C (silkworm feeding temperature) at 200 rpm. Approximately 10 μl of the mixtures were removed after 2 h for E. coli and 4 h for B. subtilis and used to separately streak an LB plate that was maintained at 37 °C for 12 h. The bacterial colonies were then counted.
Infection of Fungus
Insect pathogenic fungus Beauveria bassiana Bb13 was cultured as described previously (19). B. bassiana conidia were suspended in 0.05% Tween 20 (v/v) at a concentration of 1 × 107 conidia/ml. Larvae at the beginning of the IV-M, wandering stage, or feeding stage (V-2) were immersed in conidia suspensions and treated as described previously (19). Larvae at the same age immersed in 0.05% Tween 20 (v/v) solution were treated as a negative control. During each molting stage (determined according to the appearance change of naïve larvae), old cuticles were removed manually and suspended in PBS buffer to count hyphae. Each larva or pupa was bled to collect all hemolymph for counting hyphae. Hyphae were observed and counted using a microscope. The hemolymph volume was calculated as described (20) to determine the total number of hyphae.
Quantitative RT-PCR and Western Blot Analysis
Proteins identified in molting fluids were analyzed on a transcription level at different developmental stages as described previously (21). Primers are listed in supplemental Table S1. For the Western blot assay, ∼30 μg of cell lysate proteins or 30 μg of molting fluids were loaded per lane. Antibodies against the silkworm prophenoloxidase (a gift from Dr. T. Asano; 1:5,000) (22), M. sexta β-GRP2 (a gift from Dr. M. Kanost; 1:2,000) (23), T. castaneum CDA1 (a gift from Dr. R. W. Beeman; 1:1,000) (7), or silkworm 30Kc19 (a gift from Dr. S-Q. Xu; 1:3,000) (24) were separately used as the primary antibodies, and the AP-conjugated goat anti-rabbit IgG (1:5,000) was used as the second antibody.
RNA Interference
Tribolium was chose for screening the molting proteins to regulate ecdysis by RNAi. Genes corresponding to molting fluid proteins were chosen to BLAST the Tribolium genome. When the protein similarity was above 30%, the gene was selected for RNAi in Tribolium, which is summarized in supplemental Table S2. The sequences of all proteins are listed in supplemental Fig. S9. The primers used for gene cloning and dsRNA production are listed in supplemental Table S3. dsRNA was produced as described (10). dsRNA of each selected gene (500 ng) was injected into the dorsal side of the first or second abdominal segment of each penultimate instar larva and pharate pupa (n = 35) following the standard protocol (25). dsRNA against bacterial malE was injected as a negative control (26). The dsRNA-treated Tribolium larvae and pupae were maintained at 30 °C. On each day, phenotypes were observed. Samples were also collected for tissue section or RT-PCR to check the efficiency of RNAi on day 4 postinjection. The Tribolium ribosomal protein-6 (TcRpS6) gene was selected as the internal control (27). Primers for RT-PCR assay after RNAi in Tribolium are listed in supplemental Table S4.
RESULTS
Natural Defects in Ecdysis Accompany Melanization
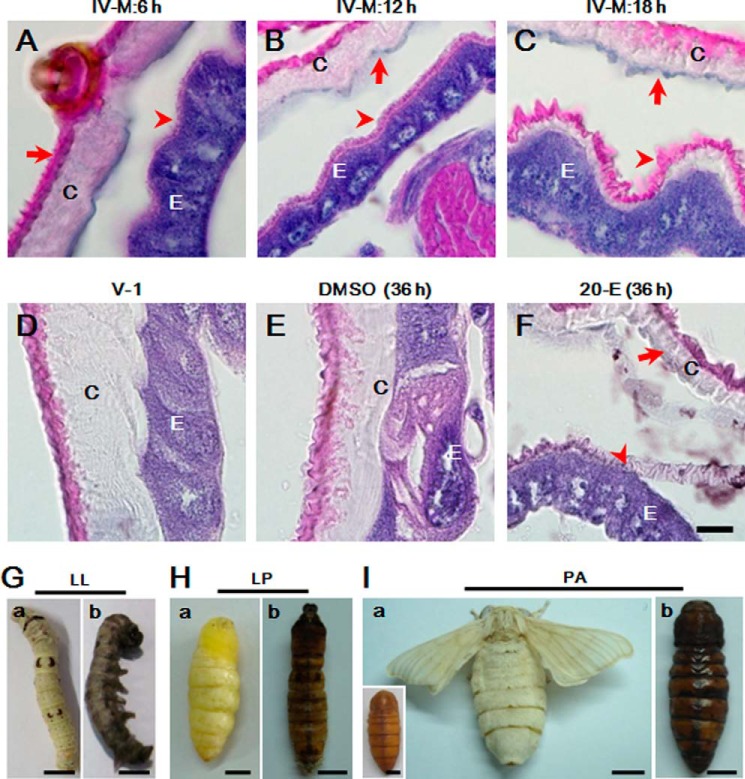
During their life cycle, Lepidoptera insects experience several larval-larval moltings. But larval-pupal or pupal-adult molting occurs only once. Tissue sectioning assays showed that a very thin layer of new waxlike material, stained red by eosin, is produced during the early fourth larval-larval molting stage (Fig. 1, A–C). New cuticle (negatively stained by eosin) produced later is still very thin even as ecdysis approaches (Fig. 1C). The cuticle does not get thicker until 2 days after feeding (Fig. 1D). When 20-E was injected into the silkworm larvae (V-0), new cuticle was produced at 36 h compared with the DMSO control injection (Fig. 1, E and F).
FIGURE 1.
Unsuccessful insect ecdysis accompanying melanization. Morphological changes of integuments on the 4th larval molting stage was performed (A–C). Waxlike matter that can be stained red by eosin was first produced (A and B), and cuticle was then produced in the later molting stage (C). Two hours after 18 h of IV-M (IV-M: 18 h), larval-larval ecdysis will happen. The layer of cuticle becomes thicker during the progress of the feeding stage (D). Compared with DMSO injection (E), injection of 20-E can induce the production of new cuticle (F). The arrows indicate the position of old cuticle (OC) that is eventually shed, and the arrowhead indicates new cuticle (NC) deposited on epidermal cells. Silkworms showed three types of ecdysis: larval-larval (LL; G), larval-pupal (LP; H), and pupal-adult (PA; I). Some silkworms could not shed the old cuticle, after which melanization was observed all over the body. a, normal ecdysis; b, unsuccessful ecdysis. The inset in I (a) represents a normal pupa before eclosion. C, cuticle; E, epidermis. IV-M: X h, X h after the beginning of the fourth molting stage. Scale bar, 20 μm (A–F) or 0.5 cm (G–I).
Insects sometimes show natural defects in ecdysis due to unknown reasons. Some silkworm larvae or pupae that fail to shed their old cuticles become melanized over the bodies (Fig. 1, G and H). We found later that melanization mainly happens in molting fluids (data not shown). Insect melanization is induced by the immunity protein prophenoloxidase (PPO) after activation (28). When larvae undergoing ecdysis were intentionally exposed to pathogenic fungi on their surface, similar melanization was observed (see below). Thus, molting fluids may have unknown factors to provide pharate instar insects protection and regulation of ecdysis. It is necessary to obtain more information on the proteomics of molting fluids in order to gain a better understanding of their potential functions.
Molting Fluid Is a Mixture Containing Many Unknown Proteins
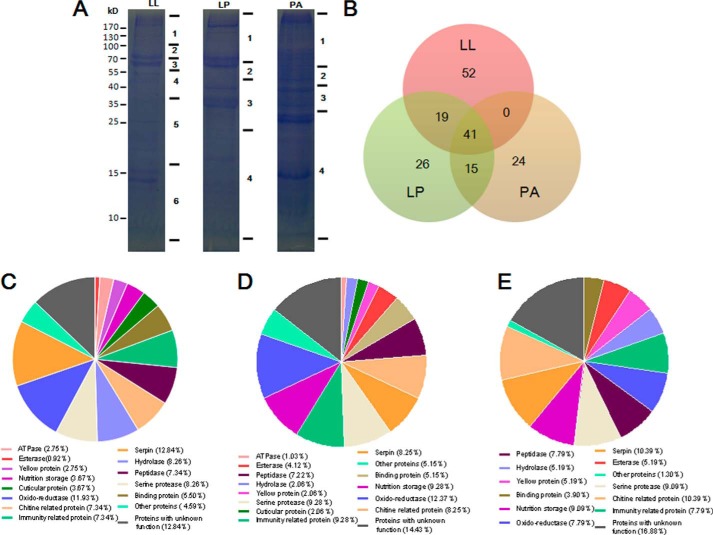
To characterize the proteome, molting fluids from the fourth larval-larval (LL), larval-pupal (LP), and pupal-adult (PA) molts were collected for LC-MS/MS assays (Fig. 2A). A total of 109 proteins were detected from larval-larval, 97 proteins from larval-pupal, and 77 proteins from pupal-adult molting fluids (Fig. 2B and supplemental Tables S5 and S6). A total of 41 proteins were found in all three types of molting fluids. There were 51, 25, and 23 proteins uniquely detected in larval-larval, larval-pupal, and pupal-adult molting fluids, respectively. Some molting proteins tightly bound to the shed cuticle (29). If the amounts of certain molting proteins were very low, we may not have been able to detect those signals. Thus, it is not surprising to see that there were differences in what molting proteins were identified. Approximately 30% of proteins were enzymes, among which two peptidases and chitinases had been purified and characterized previously (3). All others are unknown so far. The above identified proteins were classified into several groups according to their general functions (Fig. 2, C–E). These results demonstrated that there are many more proteins in molting fluids than we previously realized.
FIGURE 2.
Protein identification in molting fluids. A, approximately 20 μg of each type of molting fluid was separated on 12% SDS-polyacrylamide gels. Each gel lane was cut into several bands as indicated for LC-LC/MS assays. B, comparison of the number of proteins identified in each type of molting fluid. The overlap shows entries with the proteins from two or three groups. C–E, gene ontology categories of proteins identified in the indicated molting fluids.
In order to verify the quality of the proteomics assay and exclude contamination from hemolymph, several enzymes or proteins were selected to assess their distribution among molting fluid, integument, and plasma using a native gel or Western blot assay. Superoxide dismutase (SOD), catalase, and several types of esterases were found in different molting fluids (supplemental Tables S5 and S6). In-gel enzyme activity assays demonstrated that SOD (supplemental Fig. S2A), catalase (supplemental Fig. S2B), and esterase (supplemental Fig. S2C) have different activities and patterns in the molting fluids and integuments at different developmental stages. SOD and catalase in molting fluids are different molecules from those in the integument and plasma (supplemental Fig. S2, A and B). Some molting proteins like esterase and protease are enriched between the old and new cuticles during ecdysis but with a very small amount in integument and plasma (supplemental Fig. S2, C and D). Molting proteins like SOD, catalase, esterase, and proteases are specifically different from integument tissues and plasma, which indicates that they are specifically expressed and secreted into the molting fluids. Western blot assays confirmed the identification of 30Kc19 (supplemental Fig. S2E and Tables S5 and S6). High levels of 30Kc19 were found in integument tissues during the wandering and pupal stages. Chitin deactylase was found mainly in the pupal-adult molting fluid and less so in larval-larval and larval-pupal molting fluids (supplemental Fig. S2F). Chitin deactylase was also identified in the integument but not in the plasma. Thus, no hemolymph contamination appeared to have occurred when molting fluids were collected.
Molting Fluid Has an Independent Immunity System to Protect Pharate Instar Insects
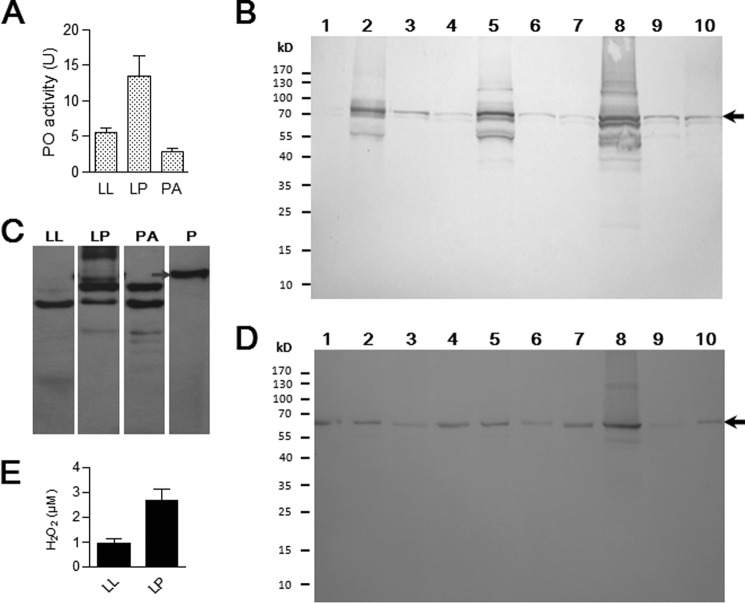
The thin and tender cuticle is easily damaged and invaded by pathogens after ecdysis. The shed old cuticle alone did not induce immunity responses for defense against microorganisms (data not shown). Molting fluid appears to have protective functions based on the presence of immunity-related and other detoxification proteins (Fig. 2, C–E, and supplemental Tables S5 and S6). When the molting fluid was collected into a tube, it became melanized within a short time frame. In insects, PO-induced melanization showed a broad spectrum of antimicrobial activity (11, 30). PPO activation is regulated by pattern recognition receptors (βGRP, PGRP, and C-type lectin) and serine protease inhibitors (serpins) (31). Prophenoloxidase-activating enzyme directly cleaves and activates PPO in the silkworm (32). Prophenoloxidase-activating enzyme was found in the larval-larval molting fluid. In addition, α-chymotrypsin can cleave PPO, resulting in PO activity (33, 34), and many molting proteases may also work to cleave PPO to produce PO. Many serpins also probably play a part in the regulation of PPO activation. Larval-pupal molting fluid showed higher PO activity than larval-larval and pupal-adult molting fluids (Fig. 3A). PPO was present in all types of molting fluids and integuments (Fig. 3B). When molting fluids were placed at room temperature for 30 min, PPO was cleaved and degraded into smaller bands (Fig. 3C). High amounts of βGRP-3 were identified in all types of molting fluids (Fig. 3D). Reactive oxygen species (ROS) are produced during melanization (35). The freshly collected molting fluids had large amounts of H2O2 that is toxic to microbes (Fig. 3E). Following the catalysis by molting heme peroxidase, H2O2 and Cl− should be synthesized into the insecticide HOCl (36). Two C-type lectins (CTL-10 and BGIMGA003660), hemolin, apolipophorin III, and lysozyme were found in different molting fluids, with known roles in immunity against microorganisms (31, 37–39). C-type lectins can detect Gram-negative bacteria and induce PPO activation (31, 40). Protease inhibitors can also prevent pathogen invasion by inhibiting pathogen-derived protease activities (3, 41, 42). Insects undergoing ecdysis have accumulated many immunity proteins and other enzymes in molting fluids, probably protecting pharate instar insects from infection.
FIGURE 3.
Prophenoloxidase activation cascade in the molting fluid. A, PO activities in larval-larval (LL), larval-pupal (LP), and pupal-adult (PA) molting fluids. Equal amounts of each molting fluid (10 μg) were used for PO activity assays. B–D, detection of PPO and βGRP-3 in molting fluids and integuments. Approximately 30 μg of cell lysates from integuments, various types of molting fluids, or 0.5 μl of plasma (V-2) was loaded per lane. In B–D, the arrow indicates the position of PPO (B and C) or βGRP-3 (D). Samples were the same as in supplemental Fig. S2. In C, molting fluids were kept at room temperature for 30 min before Western blot. E, H2O2 detected in molting fluids. Bars represent the mean of three independent measurements ± S.E. (error bars).
Inhibition on Bacterial Growth by Molting Fluids
Fresh E. coli (Gram-negative) or B. subtilis (Gram-positive) was mixed with different types of filtered molting fluids and cultured at 25 °C (optimal silkworm temperature). Colonies of microorganisms were counted, and the results showed that all molting fluids significantly inhibited Bacillus growth compared with the control (Fig. 4A). Likewise, the number of E. coli colonies decreased after incubation with different types of molting fluids (Fig. 4B). Therefore, immunity proteins in molting fluids can protect pharate instar insects by inhibiting bacterial growth.
FIGURE 4.
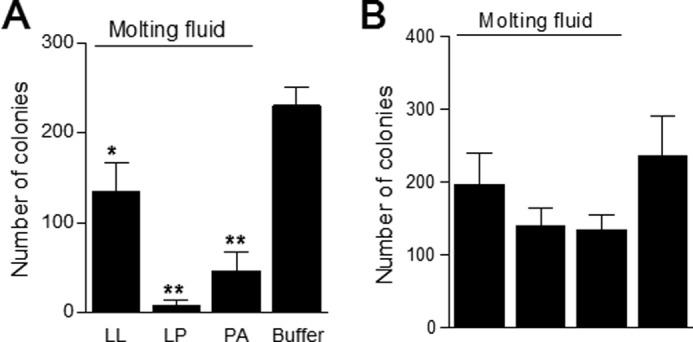
Inhibition of bacterial growth by molting fluids. Each type of molting fluid was incubated separately with fresh B. subtilis (A) or E. coli (B) for 2 or 4 h, respectively. Bacterial colonies were then counted after culture on LB plates at 37 °C for 12 h. Bars represent the mean of independent measurements ± S.E. (error bars) (n = 5 (A) and n = 4 (B)). *, p < 0.05; **, p < 0.0001.
B. bassiana Cannot Escape the Immunity in the Molting Fluids
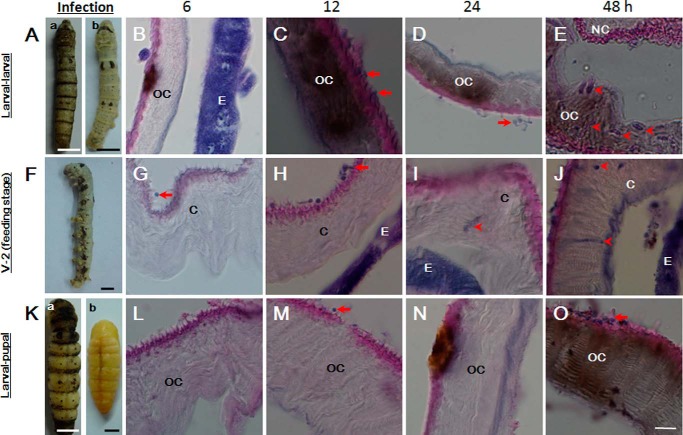
It is well understood that B. bassiana can escape the immune response of feeding larvae (43). The conidia of B. bassiana were applied to larvae during initiation of the fourth larval-larval or larval-pupal molting stage or feeding stage (V-2), as described previously (19). After fungal conidia were applied, insects undergoing larval-larval ecdysis were systematically melanized over the body at 12 h (Fig. 5A (a)). However, larvae were still intact if the melanized cuticle was manually removed (Fig. 5A (b)). A tissue section assay showed that partial melanization of the old cuticle happened at 6 h after conidia application (Fig. 5A). After 12 h, the old cuticle was systematically melanized (Fig. 5B). At 24 h, some germinated hyphae were observed on the skin (Fig. 5D), and at this time, the uninfected larvae underwent ecdysis. After 48 h, large amounts of hyphae were entrapped inside the melanized old cuticle of the few larvae that did not undergo successful ecdysis (Fig. 5E).
FIGURE 5.
Delayed germination of B. bassiana on the skins of larvae undergoing ecdysis. B. bassiana conidia were applied to the surfaces of larvae undergoing ecdysis or on feeding stages and tissue were sectioned. A–E, surface application of B. bassiana to larvae undergoing the 4th larval-larval ecdysis. Conidia germination was delayed until 24 h postapplication, but systematic melanization occurred quickly. F–J, application of B. bassiana to feeding larvae (V-2). Conidia germination was observed between 6 and 12 h. Melanization occurred after hyphae passed through the cuticle. K–O, surface application of B. bassiana to larvae undergoing larval-pupal ecdysis. Conidia germination was delayed until 48 h. In A and K, insects at different molting stages became melanized after B. bassiana application (a), and the same insects are shown after the old cuticles were manually removed (b). The arrows indicate the conidia (C, G, and M) and newly germinated hyphae (D, H, and O) on the surfaces of larvae. The arrowheads indicate hyphae inside the cuticle (E, I, and J). OC, old cuticle of ecdysis larvae; NC, new cuticle of ecdysis larvae; C, cuticle of feeding larvae; E, epidermal cells. Scale bar, 10 μm.
If conidia were applied to feeding larvae (V-2), some melanized spots were observed on the skin at 24–36 h (Fig. 5F), after which the larvae gradually died. After 6 h, the conidia were able to attach to the cuticle, and some conidia began to germinate on the skin surface (Fig. 5G). After 12–24 h, hyphae broke through the cuticles (Fig. 5, H and I), and many hyphae were already observed in the hemolymph 30 h postinfection. After 48 h, a few hyphae were observed in the slightly melanized cuticle (Fig. 5J).
For larvae receiving fungal conidia during the larval-pupal ecdysis, systematic melanization occurred at 48 h (Fig. 5, K (a) and O). Conidia did not efficiently attach to and germinate on the surface during tissue fixation (Fig. 5, L and N). Only a few melanized spots occur on the skin at ∼24 h (Fig. 5N). Significant melanization over the body was observed at ∼48 h when the normal larvae were undergoing ecdysis to become pupae (Fig. 5O), and freshly germinated B. bassiana hyphae were observed at 48 h (Fig. 5O). For pupae undergoing pupal-adult ecdysis, B. bassiana conidia could not penetrate the thick and hard cuticle to induce any change to the skin and molting fluids (data not shown).
After B. bassiana application, for melanized larvae that showed difficulty during ecdysis (∼10%), old cuticles were removed manually, and the pharate instar insects appeared normal (Figs. 5 (A (b) and K (b)) and 6A). Additionally, these larvae developed normally to become pupae and adults. No fungal cells were detected in the hemolymph of pharate instar insects (Fig. 6B). Some hyphae were observed after the old cuticles were suspended in buffer, but the numbers were significantly lower than the total number of hyphae found in hemolymph of feeding larvae at 30 h (Fig. 6B). Most hyphae collected from the manually removed melanized cuticles were in the process of dying (Fig. 6C). Some hyphae collected from the surfaces of feeding larvae were also dying. However, the number of dead fungal cells was significantly lower than that collected from old cuticles during the fourth molting stage, and it was also lower than that from larvae undergoing larval-pupal ecdysis (Fig. 6D).
FIGURE 6.
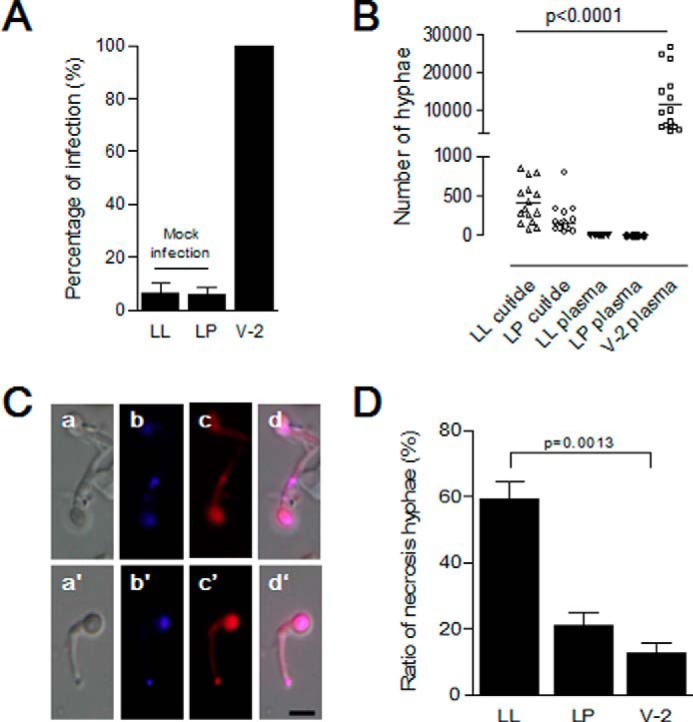
B. bassiana hyphae cannot penetrate the new cuticles of larvae undergoing ecdysis. Insect larvae undergoing the fourth larval-larval (LL) or larval-pupal (LP) ecdysis or feeding stage (V-2) were infected with B. bassiana conidia as in Fig. 5. A, percentages of infected larvae. Bars represent the means of three independent measurements ± S.E. (error bars) (15 individuals in each group). B, no hyphae were found in the hemolymph of larvae undergoing ecdysis. Hyphae on the insect surface and in molting fluids were suspended in buffer for counting after self-ecdysis or manual removal. Hyphae in hemolymph of all larvae were also counted after bleeding. Bars represent the mean of three independent measurements ± S.E. (error bars) (15 individuals in each group). C, morphology of fungal cells separated from molting fluids and cuticle surfaces. After suspension, hyphae were stained to detect living and dead cells, respectively (58, 59). Hyphae with strong red fluorescence were dead. a–d, hyphae from infected larvae (LL) as in Fig. 5A (a); a′–d′, hyphae from infected larvae (LP) as in Fig. 5K (a). d and d′, merged image using blue (b and b′) and red (c and c′) filters. D, percentages of dead hyphae as shown in C. For infected feeding larvae in V-2, hyphae washed from cuticle surfaces alone were assayed. Bars represent the means of three independent measurements ± S.E. (error bars) (15 individuals in each group). Scale bar, 5 μm.
When the shed old cuticle alone was mixed with B. bassiana conidia, no responses like melanization occurred (data not shown). Non-shed cuticle alone does not have immunity protection because B. bassiana can pass through the cuticles of feeding larvae without inducing severe melanization (Fig. 5, G–I). These data indicate that B. bassiana can escape integument immunity in feeding larvae. However, larvae undergoing larval-larval and larval-pupal ecdysis can detect fungi and respond by delaying B. bassiana conidia germination on the surfaces, and systematic melanization in the molting fluid and skin of larvae undergoing ecdysis was triggered at different times to prevent further infection. Therefore, the molting fluids, which accumulate among the old and new cuticles, have important roles in protecting pharate instar insects.
Successful Ecdysis Is the Result of Systematic Regulation by Molting Proteins
When molting fluid was removed from the prepupae of silkworms, defects in ecdysis were typically observed (supplemental Fig. S3). In Tribolium, several chitinolytic proteins affect ecdysis after RNAi knockdown (5–7). The above chitinolytic proteins have also been found in silkworm molting fluids (supplemental Tables S5 and S6 and Fig. S4A), indicating common molting fluid proteins between the silkworm and Tribolium. Therefore, Tribolium was selected to identify genes that regulate ecdysis using RNAi. To examine protein expression in the integument during each molting stage, transcriptional changes in selected genes of the silkworm were assayed. Injection of 20-E can induce the production of new larval cuticle (supplemental Fig. S1E); thus, the influence of 20-E on gene transcription was also assessed.
Chitin Recycling Affects Ecdysis
During ecdysis, the amount of chitin was lower in the old cuticle than in the new cuticle (supplemental Fig. S1, A–C), indicative of degradation of the old cuticle (3). Chitinase activities in the melanized molting fluids induced by fungal exposure were lower than those in normal molting fluids (supplemental Fig. S4B). In addition, some melanized larvae showed defects in shedding of the old cuticle (Figs. 5 (A and K) and 6A). The decreased chitinase activities in melanized molting fluids from larvae having difficulty in ecdysis suggest that normal chitinase activities are important for successful ecdysis in insects, which have been studied in Tribolium (5–7).
Protein Recycling Is a Prerequisite for Normal Ecdysis
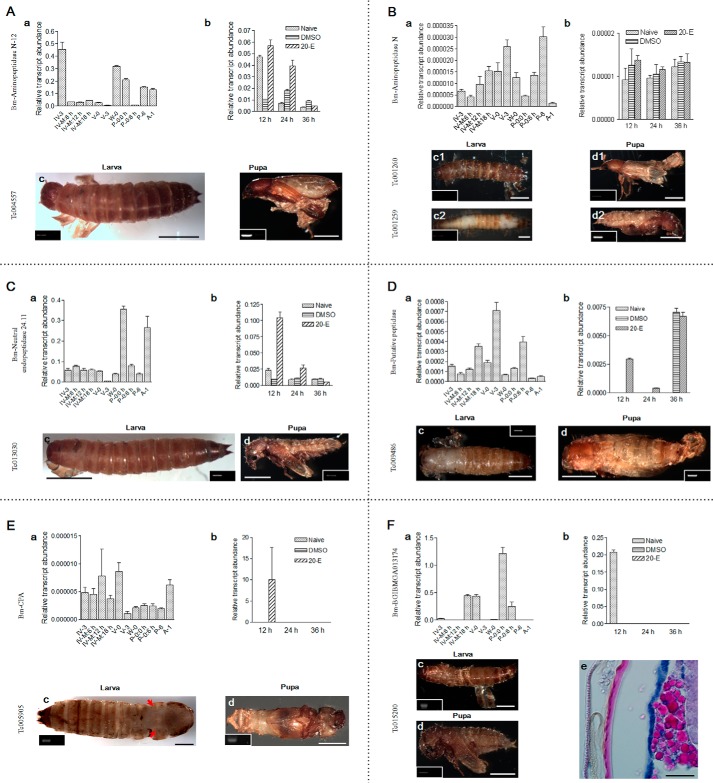
In molting fluids, several peptidases that cleave and degrade proteins at different peptide sequences were identified (supplemental Table S5). Their influence on ecdysis remains unknown. The patterns of molting fluid proteases differ between epidermal cells and plasma (supplemental Fig. S2D). All six peptidases were transcribed in epidermal cells at different stages (Fig. 7, A (a) to F (a)). Aminopeptidase N, a putative peptidase, and carboxypeptidase A were highly transcribed during the feeding stage. Other peptidases were highly expressed during different stages of ecdysis. The injection of 20-E can regulate the transcription of aminopeptidase N-12 (Fig. 7A (b)), aminopeptidase N (Fig. 7B (b)), neutral endopeptidase (Fig. 7C (b)), and carboxypeptidase A (CPA; Fig. 7E (b)), but not the putative peptidase or BGIBMGA013174 (a zinc metalloprotease), after 12 and/or 24 h (Fig. 7, D (b) and F (b)).
FIGURE 7.
Peptidases in molting fluids affect ecdysis. a, transcription of the indicated genes was assayed in integuments at different developmental stages. b, influence of 20-E on gene transcription. Larvae receiving DMSO injection and naive larvae were used as controls. c and d, typical phenotypes of new larvae (c) or adults (d) were observed when Tribolium larvae or pharate pupae, respectively, were injected with dsRNA to knock down the corresponding gene as indicated. RT-PCR was performed to assess the knockdown efficiency of RNAi, which is shown as an inset in the corresponding phenotype. The loading control for each treatment is presented in supplemental Fig. S9. Bm, B. mori; Tc, T. castaneum. All information is the same with the following assays unless otherwise specified. Silkworm molting peptidases, including aminopeptidase N-12 (A), aminopeptidase N (B), neutral endopeptidase (C), putative peptidase (D), carboxypeptidase A (CPA) (E), and BGIBMGA013174 (F) were selected to examine expression at different developmental stages (a) and the influence of 20-E (b). The corresponding genes indicated were knocked down in Tribolium to examine their influence on ecdysis. F (e) shows a Tribolium larva tissue section undergoing unsuccessful ecdysis. From left to right, the old cuticle (left), new cuticle (red), and epidermal cells (blue) are shown. Bar, 0.1 cm (10 μm in F (e)).
Aminopeptidase N has two corresponding genes in Tribolium that were also examined. When the corresponding gene of each peptidase was knocked down in Tribolium larvae, the larvae could not shed their old cuticle in the absence of manual assistance to expose the old cuticle (panels c, c1, and c2 in Fig. 7, A–F). When neutral endopeptidase (Fig. 7C (c)), the putative peptidase (Fig. 7D (c)), and BGIBMGA013174 (Fig. 7F (c)) were knocked down separately, we observed defects in larval head capsule slippage. Knockdown of aminopeptidase N (Fig. 7B, c1/c2) or the putative peptidase (Fig. 7D (c)) induced a defect in cuticle sclerotization.
Each peptidase gene was also knocked down in Tribolium pupae, which affected adult ecdysis and development. The primary phenotype was a defect in old cuticle shedding in new adults (panels d, d1, and d2 in Fig. 7, A–F). When neutral endopeptidase was knocked down in pupae, new adults remained covered in old cuticle without obvious head capsule slippage (Fig. 7C (d)).
Another carboxypeptidase was detected in pupal-adult molting fluid (supplemental Tables S5 and S6), the transcription level of which was also obvious in the pupal stage (supplemental Fig. S6a). However, 20-E had no obvious effects on transcriptional changes (data not shown). When the corresponding gene in Tc015782 was knocked down in Tribolium pupae, the majority of old cuticle was shed from the head and body but not from the wings (supplemental Fig. S6b).
When the corresponding gene of BGIBMGA013174 was knocked down in Tribolium larvae, tissue sectioning showed two layers of cuticle (Fig. 7F (e)). The new cuticle was produced normally; however, the old cuticle had lost the region that stained positive for eosin. Thus, the old cuticle may have been degraded by molting enzymes. These results demonstrate that these peptidases are very important for successful ecdysis.
Regulation of ROS Is Important for Ecdysis
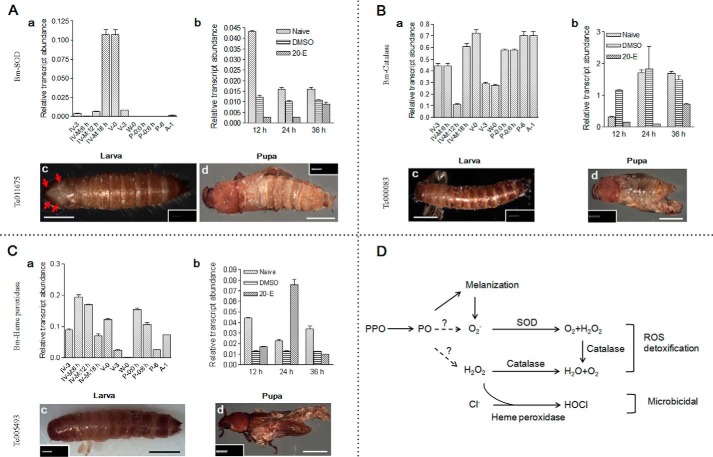
SOD, heme peroxidase, and catalase that were found in molting fluids (Fig. 3E) have close relationship with ROS degradation (36, 44). Transcription of these three genes in integument was higher toward the end of the larval-larval molting stage (Fig. 8, A (a), B (a), and C (a)). 20-E could positively regulate heme peroxidase, but not SOD and catalase, 24 h postinjection (Fig. 8, B (b) and C (b)).
FIGURE 8.
Enzymes regulating reactive oxygen species are essential to ecdysis. SOD (A), catalase (B), and heme peroxidase (C) were identified in silkworm molting fluids (supplemental Tables S5 and S6). The corresponding genes were knocked down in both Tribolium larvae and pupae. D, a summary of SOD, catalase, and heme peroxidase regulation of ROS production during melanization. Scale bar, 0.1 cm.
When the corresponding genes in Tribolium were knocked down in larvae and pupae, respectively, the old cuticles could not be shed. Head capsule slippage occurred in larvae transfected with SOD RNAi (Fig. 8A (c)) but failed in larvae transfected with heme peroxidase or catalase RNAi (Fig. 8, B (c) and C (c)). The sclerotization of new larval cuticle was successful in all treatments. When pupae were injected with dsRNA to knock down SOD (Fig. 8A (d)) or catalase (Fig. 8B (d)), we observed defects in adult development. For example, adults had old cuticle on their bodies and wings. In addition, when heme peroxidase was knocked down in pupae, the adults remained covered in old cuticle (Fig. 8C (d)). In Caenorhabditis elegans, animal heme peroxidase MLT-7 regulates each larval molting stage by influencing collagen cross-linking (45). Based on these results, there may be similar or even unknown mechanisms that regulate ecdysis for three ROS-related proteins.
Detoxification Is Indispensable to Ecdysis
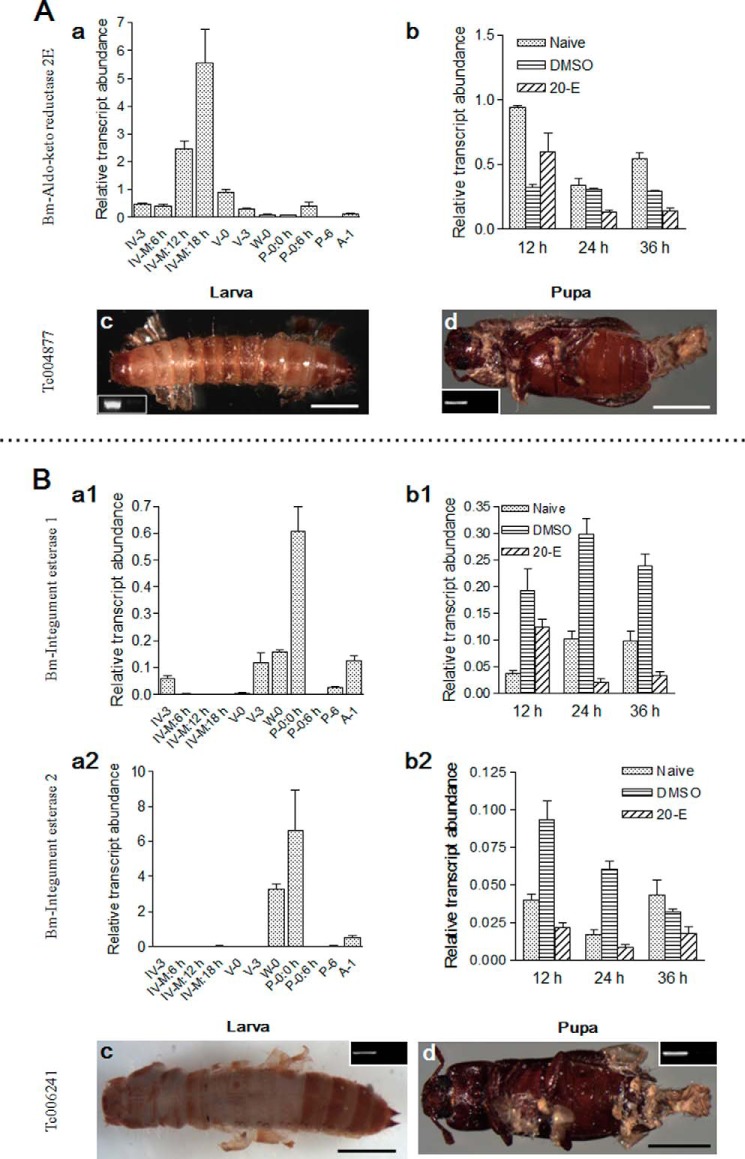
Aldo-keto reductase 2E exerts detoxification functions via the oxidization of small molecules and pollutants (46). Two silkworm integument esterases were encoded by one gene in Tribolium. Integument esterases (IE1 and IE2) can degrade pheromones and detoxify xenobiotics (47). The corresponding genes in Tribolium were knocked down in larvae and pupae, respectively. In the silkworm, aldo-keto reductase 2E was strongly expressed toward the end of larval-larval molting but weaker during other molting stages (Fig. 9A (a)). IE1 and IE2 were highly expressed during larval-pupal molting but weaker during other molting stages (Fig. 9B (a1 and a2)). However, they were not regulated by 20-E injection (Fig. 9, A (b) and B (b1 and b2)). When two groups of genes were knocked down in larvae, no head capsule slippage was observed (Fig. 9, A (c) and B (c)). Aldo-keto reduction 2E did not affect the sclerotization of larval cuticle (Fig. 9A (c)), whereas integument esterases did (Fig. 9B (c)). When integument esterases were knocked down in pupae, adults developed normally; however, the old cuticles did not shed from some of the adults (Fig. 9, A (d) and B (d)). Molting fluid is a small system containing many proteins that are involved in recycling of old cuticles and other physiological functions, which may produce a lot of toxic molecules. The new integuments are extremely thin (Fig. 1, A–C). When the detoxification proteins are lost by RNAi, those toxic molecules may enter the hemocoel to interrupt ecdysis and development. However, proof of this conclusion still requires further work.
FIGURE 9.
Two detoxification genes influence ecdysis. Aldo-keto reductase 2E (A) and two integument esterases (B) were identified in silkworm molting fluids. A, the gene corresponding to aldo-keto reductase 2E in Tribolium was knocked down in both larvae and pupae. B, two integument esterases in the silkworm were assayed, respectively. Tribolium contains a gene corresponding to two silkworm integument esterases. Scale bar, 0.1 cm.
Balance of Molting Fluid Pressure Guarantees Successful Ecdysis
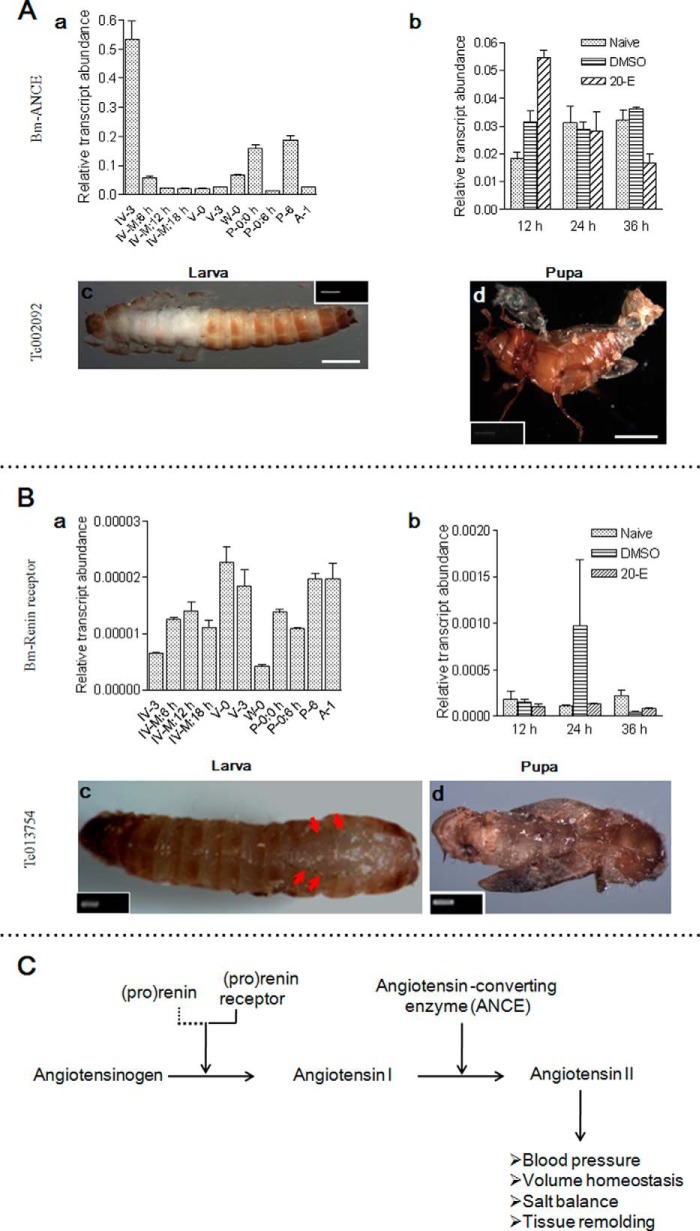
The renin-angiotensin system regulates blood pressure and fluid balance in humans (48). The angiotensin-converting enzyme (ANCE)-related gene and rennin receptor were also identified in molting fluids (supplemental Tables S5 and S6). ANCE transcription occurs at different times (Fig. 10A (a)). 20-E increased its transcription at 12 h postinjection (Fig. 10A (b)). When ANCE was knocked down, new larvae and adults could not shed their old cuticles (Fig. 10A (c and d)). In addition, the new larval cuticle showed defects in sclerotization.
FIGURE 10.
The renin-angiotensin system is essential to ecdysis. ANCE (A) and the renin receptor (B) were detected in silkworm molting fluids. The corresponding genes indicated were knocked down in both Tribolium larvae and pupae. ANCE and the renin receptor affect ecdysis. C, summary of how the renin-angiotensin system regulates blood pressure and fluid balance in animals (48, 60). Renin was not identified in insects, as indicated by the dotted line. Scale bar, 0.1 cm.
The renin receptor was detected in larval-larval molting fluid alone (supplemental Table S5 and S6). However, this gene was transcribed at different times (Fig. 10B (a)), and 20-E did not significantly alter its expression (Fig. 10B (b)). The corresponding gene was knocked down in Tribolium larvae and pupae, respectively. Larvae showed normal head capsule slippage (Fig. 10B (c)). Old cuticle had been removed from the adult head but remained covering other regions (Fig. 10B (d)).
In this system, active renin cleaves angiotensinogen into angiotensin I, which is then cleaved into angiotensin II by ANCE (Fig. 10C). Renin binding to its receptor can increase renin activity significantly (48). Renin is a protease with cleavage activity (48). However, neither renin nor renin-like genes have been identified at this time; thus, specific proteases in the molting fluids may function as renin. In C. elegans, ANCE also affects molting after knockdown (49). Insects may use the components of the renin-angiotensin system to balance the molting fluids and pressure, which deserves further study.
Signal Transfer Regulates Ecdysis
Several binding proteins were identified in molting fluids, and signals may be transferred among molting fluids, epidermal cells, and other tissues. Peptidylprolyl isomerase B is a cyclosporine-binding protein located in the endoplasmic reticulum, and this protein has a close association with the secretory pathway and can be released into biological fluids (50). Peptidylprolyl isomerase B is transcribed at different stages (Fig. 11A (a)) and is also regulated by 20-E at 12 h postinjection (Fig. 11A (b)). When the corresponding gene in Tribolium was knocked down in larvae and pupae, respectively, the shedding of old cuticle was prevented (Fig. 11A (c and d)). There was a defect in larvae head capsule slippage, but the new cuticle was sclerotized (Fig. 11A (c)). The old cuticle had been removed from the heads but remained covering other body parts in adults (Fig. 11A (d)). Thus, peptidylprolyl isomerase B can also affect ecdysis.
FIGURE 11.
A binding protein and three proteins with unknown functions influence ecdysis. Peptidylprolyl isomerase B (A), BGIBMGA011399 (B), BGIBMGA003112 (C), and BGIBMGA012766 (D) were identified in silkworm molting fluids. The corresponding genes indicated were knocked down in both Tribolium larvae and pupae. The arrow (C) indicates new larva inside the old cuticle. Scale bar, 0.1 cm.
Gelsolin is an actin-binding protein, and regucalcin is a calcium-binding protein. The injection of 20-E affected gelsolin, but not regucalcin, transcription at 24 and 36 h (supplemental Fig. S7A (b)). After knockdown, larvae showed defects in head capsule slippage (supplemental Fig. S7, A (c) and B (c)). In addition, adult development was defective (supplemental Fig. S7, A (d) and B (d)). Based on these results, signal transfer appears important for normal ecdysis.
Proteins with Unknown Functions Affect Ecdysis
BGIBMGA011399, BGIBMGA012766, and BGIBMGA003112, whose functions are unknown, were each identified in different types of molting fluids (supplemental Tables S5 and S6). BGIBMGA011399 and BGIBMGA003112 were significantly transcribed in integuments at different stages (Fig. 11, B (a) and C (a)). The transcription of BGIBMGA012766 was highest on P-6 (Fig. 11D (a)). BGIBMGA011399 expression was not significantly affected by 20-E injection (Fig. 11B (b)). However, BGIBMGA003112 and BGIBMGA012766 were significantly transcribed at 24 and 12 h postinjection of 20-E, respectively (Fig. 11, C (b) and D (b)). When the corresponding genes in Tribolium were knocked down in larvae and pupae, old cuticles could not be shed from larvae or adults, respectively. All new larvae showed defects in head capsule slippage (Fig. 11, B (c), C (c), and D (c)). When the gene encoding BGIBMGA003112 was knocked down, new larvae were clearly observed under the old cuticle (Fig. 11C (c)). Knockdown of the gene corresponding to BGIBMGA012766 affected the sclerotization of new cuticle (Fig. 11D (c)). When the corresponding three genes were knocked down in pupae, they all induced abnormal adult development with old cuticles remaining on the new adults (Fig. 11, B (d), C (d), and D (d)).
Although detected in one type of molting fluid, three proteins with unknown functions (BGIBMGA006830, BGIBMGA001098, and BGIBMGA009750) were also assayed. In silkworm, BGIBMGA006830 and BGIBMGA001098 were transcribed at different stages (supplemental Fig. S7, C (a) and D (a)). In addition, they were not affected by 20-E treatment (supplemental Fig. S7, C (b) and D (b)). BGIBMGA009750 was detected in pupal-adult molting fluid (supplemental Tables S5 and S6), and the transcriptional changes were obvious during the pupal stage (supplemental Fig. S7E (a)). The influence of 20-E on transcription of this gene was not assessed. The corresponding genes in Tribolium were knocked down in larvae and/or pupae, respectively. Based on these results, these three genes affect adult development (supplemental Fig. S7, C (d), D (d), and E (b)). However, after their knockdown in larvae, defects in head capsule slippage resulted (supplemental Fig. S7, C (c) and D (c)).
Immunity Genes Do Not Directly Affect Ecdysis
PPO and βGRP-3 are present in molting fluids and integuments (Fig. 3, B and D). When the corresponding βGRP-3 gene in Tribolium was knocked down, no defects in ecdysis were observed (supplemental Fig. S5). PPO genes had no defects in ecdysis after being knocked down in Tribolium (10), indicating that immunity proteins in molting fluids did not affect molting but protected new life after ecdysis. In addition, certain other genes, including those encoding serine protease and serpin, did not affect molting after knockdown in Tribolium (supplemental Table S2).
All of the genes that affected insect ecdysis as identified in Tribolium are summarized in supplemental Table S2. Several genes shown previously to affect Tribolium ecdysis (5–7) with corresponding proteins in molting fluids are included in this table (supplemental Table S2 and Fig. S8). The physiological functions of protein and chitin recycling, detoxification, molting fluid balance, signals transfer, and other unknown functions, as determined by molting proteins, are important to ecdysis. Immunity proteins cannot directly affect ecdysis but are very important for protecting pharate instar insects.
DISCUSSION
Successful ecdysis is important for development, growth, and metamorphosis in insects (3, 51, 52). Molting and ecdysis are promoted by 20-E (52). Under the regulation of 20-E and juvenile hormone, many molting proteins with different functions are expressed and accumulated between the new and old cuticles. As summarized in Fig. 12, normal and successful ecdysis requires all molting proteins to work together for nutrition cycling, detoxification, signal transfer, fluid pressure balance, and immune protection. When insects are infected by pathogens during ecdysis, PPO is activated in the molting fluid, causing rapid systematic melanization over the body (Fig. 5, A (a) and K (a)). In the melanized molting fluids, chitinase activities were decreased (supplemental Fig. S4B), which may affect the shedding of old cuticles in some melanized silkworm larvae according to previous reports (5–7). However, the pharate instar insects under the old cuticles are protected from further infection. This process of melanized ecdysis is induced by pathogens. However, pharate instar insect development was not affected due to immunity protection in the molting fluids (Figs. 4–6). The expression of certain genes may be interrupted by the application of pesticides (53) or even RNAi, which can inhibit ecdysis and even development (Figs. 7–11 and supplemental Table S2). This is indicative of unsuccessful ecdysis that may also interrupt insect development.
FIGURE 12.
Regulation of insect ecdysis. Insects undergo several types of ecdysis during a life cycle: larval-larval, larval-pupal, and pupal-adult ecdysis. During ecdysis, the increasing concentration of 20-E promotes protein expression (61) and the production of new cuticles (Fig. 1F). Many proteins in the molting fluids are indispensable and must work in coordination to induce normal ecdysis. When insects were infected by pathogens before ecdysis, PPO was activated in molting fluids, and melanization occurred (Fig. 5, A (a) and K (a)). Immune responses indirectly regulated ecdysis in some insects, according to decreased chitinase activities after melanizaton (supplemental Fig. S4B). The pharate instar insects under the melanized cuticle were intact. This melanized ecdysis is a result of immune responses to pathogens. When molting protein expression was interrupted (e.g. by RNAi or pesticide application (53)), the insect could not shed the old cuticle on time. Therefore, the insect cannot undergo successful ecdysis unless all molting proteins are expressed and work in coordination to remove the old cuticle.
In C. elegans, many genes, including those encoding transcription factors, signal proteins, and molecules involved in new cuticle production and old cuticle release, were found to affect nematode molting (49, 51). However, chitinolytic enzymes that can affect different types of ecdysis in Tribolium (5–7) do not function in nematodes. Some proteins that regulate C. elegans and Tribolium ecdysis are not found in the silkworm molting fluids. For example, TRXR-1 thioredoxin reductase is essential to C. elegans molting by reducing disulfides in cuticle (54). In Tribolium, two genes, Knickkopf (TcKnk) and Retroactive (TcRtv), protect new cuticle from degradation by molting chitinase (27, 55). When these genes were knocked down, serious defects in molting were observed. These proteins were not found in silkworm molting fluids. In M. sexta, proteins in the diet can also affect the metamorphic molt (56). All nematodes and arthropods are included among ecdysozoan animals (51). To complete metamorphosis, insects experience different stages of ecdysis before becoming next stage larvae, pupae, and adults. Nematodes, including C. elegans, require four rounds of larval ecdysis to become adults (57). Therefore, this developmental difference may be due to different mechanisms that regulate ecdysis between Nematoda and insects. In C. elegans, transcription factors also affect molting. Therefore, in the future, it is not surprising to find many factors in or outside of molting fluids that can regulate insect ecdysis. The upstream and/or downstream region of each gene or protein may also be essential for molting. Any minor defects in molting are fatal to insects, including pests (51). Therefore, such genes represent “exciting targets for pesticides,” as discussed previously (49). Based on these results, the detailed mechanisms regulating ecdysis by each molting protein evaluated in this study require further investigation.
Supplementary Material
Acknowledgments
We thank Chengshu Wang, Yongzhen Xu, Xiao-Qiang Yu, Peilin Chen, and Brenda T. Beerntsen for help, comments, and encouragement.
This work was supported by National Basic Research Program of China Grant 2012CB114605, National Natural Science Foundation of China Grant 31172151, and Ministry of Agriculture of China Grant 2011ZX08009-003.

This article contains supplemental Tables S1–S6 and Figs. S1–S9.
- 20-E
- 20-hydroxyecdysone
- PO
- phenoloxidase
- PPO
- prophenoloxidase
- SOD
- superoxide dismutase
- ROS
- reactive oxygen species
- ANCE
- angiotensin-converting enzyme.
REFERENCES
- 1. Telford M. J., Bourlat S. J., Economou A., Papillon D., Rota-Stabelli O. (2008) The evolution of the Ecdysozoa. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Truman J. W., Riddiford L. M. (2002) Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 47, 467–500 [DOI] [PubMed] [Google Scholar]
- 3. Reynolds S. E., Samuels R. I. (1996) Physiology and biochemistry of insect moulting fluid. Adv. Insect Physiol. 26, 157–232 [Google Scholar]
- 4. Muthukrishnan S., Merzendorfer H., Arakane Y., Kramer K. J. (2012) Chitin metabolism in insects. in Insect Molecular Biology and Biochemistry (Gilbert L. I., ed) pp. 193–235, Elsevier Inc., San Diego [Google Scholar]
- 5. Zhu Q., Arakane Y., Beeman R. W., Kramer K. J., Muthukrishnan S. (2008) Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 105, 6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogenkamp D. G., Arakane Y., Kramer K. J., Muthukrishnan S., Beeman R. W. (2008) Characterization and expression of the β-N-acetylhexosaminidase gene family of Tribolium castaneum. Insect Biochem. Mol. Biol. 38, 478–489 [DOI] [PubMed] [Google Scholar]
- 7. Arakane Y., Dixit R., Begum K., Park Y., Specht C. A., Merzendorfer H., Kramer K. J., Muthukrishnan S., Beeman R. W. (2009) Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 39, 355–365 [DOI] [PubMed] [Google Scholar]
- 8. Terenius O., Papanicolaou A., Garbutt J. S., Eleftherianos I., Huvenne H., Kanginakudru S., Albrechtsen M., An C., Aymeric J. L., Barthel A., Bebas P., Bitra K., Bravo A., Chevalier F., Collinge D. P., Crava C. M., de Maagd R. A., Duvic B., Erlandson M., Faye I., Felföldi G., Fujiwara H., Futahashi R., Gandhe A. S., Gatehouse H. S., Gatehouse L. N., Giebultowicz J. M., Gómez I., Grimmelikhuijzen C. J., Groot A. T., Hauser F., Heckel D. G., Hegedus D. D., Hrycaj S., Huang L., Hull J. J., Iatrou K., Iga M., Kanost M. R., Kotwica J., Li C., Li J., Liu J., Lundmark M., Matsumoto S., Meyering-Vos M., Millichap P. J., Monteiro A., Mrinal N., Niimi T., Nowara D., Ohnishi A., Oostra V., Ozaki K., Papakonstantinou M., Popadic A., Rajam M. V., Saenko S., Simpson R. M., Soberon M., Strand M. R., Tomita S., Toprak U., Wang P., Wee C. W., Whyard S., Zhang W., Nagaraju J., Ffrench-Constant R. H., Herrero S., Gordon K., Swevers L., Smagghe G. (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J. Insect. Physiol. 57, 231–245 [DOI] [PubMed] [Google Scholar]
- 9. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 10. Shao Q., Yang B., Xu Q., Li X., Lu Z., Wang C., Huang Y., Söderhäll K., Ling E. (2012) Hindgut innate immunity and regulation of fecal microbiota through melanization in insects. J. Biol. Chem. 287, 14270–14279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang P., Granados R. R. (2000) Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Mol. Biol. 30, 135–143 [DOI] [PubMed] [Google Scholar]
- 12. Samuels R. I., Reynolds S. E. (1993) Moulting fluid enzymes of the tobacco hornworm, Manduca sexta: inhibitory effect of 20-hydroxyecdysone on the activity of the cuticle degrading enzyme MFP-1. J. Insect Physiol. 39, 633–637 [Google Scholar]
- 13. Xia Q., Zhou Z., Lu C., Cheng D., Dai F., Li B., Zhao P., Zha X., Cheng T., Chai C., Pan G., Xu J., Liu C., Lin Y., Qian J., Hou Y., Wu Z., Li G., Pan M., Li C., Shen Y., Lan X., Yuan L., Li T., Xu H., Yang G., Wan Y., Zhu Y., Yu M., Shen W., Wu D., Xiang Z., group G. a., Yu J., Wang J., Li R., Shi J., Li H., Li G., Su J., Wang X., Li G., Zhang Z., Wu Q., Li J., Zhang Q., Wei N., Xu J., Sun H., Dong L., Liu D., Zhao S., Zhao X., Meng Q., Lan F., Huang X., Li Y., Fang L., Li C., Li D., Sun Y., Zhang Z., Yang Z., Huang Y., Xi Y., Qi Q., He D., Huang H., Zhang X., Wang Z., Li W., Cao Y., Yu Y., Yu H., Li J., Ye J., Chen H., Zhou Y., Liu B., Wang J., Ye J., Ji H., Li S., Ni P., Zhang J., Zhang Y., Zheng H., Mao B., Wang W., Ye C., Li S., Wang J., Wong G. K.-S., Yang H. (2004) A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306, 1937–1940 [DOI] [PubMed] [Google Scholar]
- 14. Ken C.-F., Hsiung T.-M., Huang Z.-X., Juang R.-H., Lin C.-T. (2005) Characterization of Fe/Mn-superoxide dismutase from diatom Thallassiosira weissflogii: cloning, expression, and property. J. Agric. Food Chem. 53, 1470–1474 [DOI] [PubMed] [Google Scholar]
- 15. Jebara S., Jebara M., Limam F., Aouani M. E. (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 162, 929–936 [DOI] [PubMed] [Google Scholar]
- 16. Kaji K., Tomino S., Asano T. (2009) A serine protease in the midgut of the silkworm, Bombyx mori: protein sequencing, identification of cDNA, demonstration of its synthesis as zymogen form and activation during midgut remodeling. Insect Biochem. Mol. Biol. 39, 207–217 [DOI] [PubMed] [Google Scholar]
- 17. Katzenellenbogen B. S., Kafatos F. C. (1971) General esterases of silkmoth moulting fluid: preliminary characterization. J. Insect Physiol. 17, 1139–1151 [Google Scholar]
- 18. Shen C. R., Chen Y. S., Yang C. J., Chen J. K., Liu C. L. (2010) Colloid chitin azure is a dispersible, low-cost substrate for chitinase measurements in a sensitive, fast, reproducible assay. J. Biomol. Screen. 15, 213–217 [DOI] [PubMed] [Google Scholar]
- 19. Shang Y., Duan Z., Huang W., Gao Q., Wang C. (2012) Improving UV resistance and virulence of Beauveria bassiana by genetic engineering with an exogenous tyrosinase gene. J. Invertebr. Pathol. 109, 105–109 [DOI] [PubMed] [Google Scholar]
- 20. Ling E., Shirai K., Kanekatsu R., Kobayashi Y., Tu Z., Funayama T., Watanabe H., Kiguchi K. (2003) Why does hemocyte density rise at the wandering stage in the silkworm, Bombyx mori? J. Insect Biotechnol. Sericol. 72, 101–109 [Google Scholar]
- 21. Xu Q., Lu A., Xiao G., Yang B., Zhang J., Li X., Guan J., Shao Q., Beerntsen B. T., Zhang P., Wang C., Ling E. (2012) Transcriptional profiling of midgut immunity response and degeneration in the wandering silkworm, Bombyx mori. PLoS One 7, e43769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asano T., Takebuchi K. (2009) Identification of the gene encoding pro-phenoloxidase A(3) in the fruitfly, Drosophila melanogaster. Insect Mol. Biol. 18, 223–232 [DOI] [PubMed] [Google Scholar]
- 23. Jiang H., Ma C., Lu Z. Q., Kanost M. R. (2004) β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: an acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem. Mol. Biol. 34, 89–100 [DOI] [PubMed] [Google Scholar]
- 24. Ji M. M., Liu A. Q., Gan L. P., Xing R., Wang H., Sima Y. H., Xu S. Q. (2013) Functional analysis of 30K proteins during silk gland degeneration by a caspase-dependent pathway in Bombyx. Insect Mol. Biol. 22, 273–283 [DOI] [PubMed] [Google Scholar]
- 25. Tomoyasu Y., Denell R. (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev. Genes Evol. 214, 575–578 [DOI] [PubMed] [Google Scholar]
- 26. Tan A., Palli S. R. (2008) Identification and characterization of nuclear receptors from the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 38, 430–439 [DOI] [PubMed] [Google Scholar]
- 27. Chaudhari S. S., Arakane Y., Specht C. A., Moussian B., Boyle D. L., Park Y., Kramer K. J., Beeman R. W., Muthukrishnan S. (2011) Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. U.S.A. 108, 17028–17033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ashida M., Brey P. (1998) Recent advances on the research of the insect prophenoloxidase cascade. in Molecular Mechanisms of Immune Responses in Insects (Brey P., Hultmark D., eds) pp. 135–172, Chapman & Hall, London [Google Scholar]
- 29. He N., Botelho J. M. C., McNall R. J., Belozerov V., Dunn W. A., Mize T., Orlando R., Willis J. H. (2007) Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem. Mol. Biol. 37, 135–146 [DOI] [PubMed] [Google Scholar]
- 30. Zhao P., Li J., Wang Y., Jiang H. (2007) Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem. Mol. Biol. 37, 952–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanost M. R., Jiang H., Yu X. Q. (2004) Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198, 97–105 [DOI] [PubMed] [Google Scholar]
- 32. Satoh D., Horii A., Ochiai M., Ashida M. (1999) Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori: purification, characterization, and cDNA cloning. J. Biol. Chem. 274, 7441–7453 [DOI] [PubMed] [Google Scholar]
- 33. Onishi E., Dohke K., Ashida M. (1970) Activation of prephenoloxidase: II. activation by α-chymotrypsin. Arch. Biochem. Biophys. 139, 143–148 [DOI] [PubMed] [Google Scholar]
- 34. Binggeli O., Neyen C., Poidevin M., Lemaitre B. (2014) Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 10, e1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nappi A. J., Christensen B. M. (2005) Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 35, 443–459 [DOI] [PubMed] [Google Scholar]
- 36. Ha E. M., Oh C. T., Bae Y. S., Lee W. J. (2005) A direct role for dual oxidase in Drosophila gut immunity. Science 310, 847–850 [DOI] [PubMed] [Google Scholar]
- 37. Zdybicka-Barabas A., Cytryńska M. (2011) Involvement of apolipophorin III in antibacterial defense of Galleria mellonella larvae. Comp Biochem. Physiol. B Biochem. Mol. Biol. 158, 90–98 [DOI] [PubMed] [Google Scholar]
- 38. Weers P. M. M., Ryan R. O. (2006) Apolipophorin III: role model apolipoprotein. Insect Biochem. Mol. Biol. 36, 231–240 [DOI] [PubMed] [Google Scholar]
- 39. Watanabe A., Miyazawa S., Kitami M., Tabunoki H., Ueda K., Sato R. (2006) Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph: mechanism of wide-range microorganism recognition and role in immunity. J. Immunol. 177, 4594–4604 [DOI] [PubMed] [Google Scholar]
- 40. Yu X.-Q., Gan H., Kanost M. R. (1999) Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem. Mol. Biol. 29, 585–597 [DOI] [PubMed] [Google Scholar]
- 41. Li Y., Zhao P., Liu S., Dong Z., Chen J., Xiang Z., Xia Q. (2012) A novel protease inhibitor in Bombyx mori is involved in defense against Beauveria bassiana. Insect Biochem. Mol. Biol. 42, 766–775 [DOI] [PubMed] [Google Scholar]
- 42. Sabotič J., Kos J. (2012) Microbial and fungal protease inhibitors: current and potential applications. Appl. Microbiol. Biotechnol. 93, 1351–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. St Leger R. J., Wang C. (2010) Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 85, 901–907 [DOI] [PubMed] [Google Scholar]
- 44. Diaz-Albiter H., Sant'Anna M. R., Genta F. A., Dillon R. J. (2012) Reactive oxygen species-mediated immunity against Leishmania mexicana and Serratia marcescens in the sand phlebotomine fly Lutzomyia longipalpis. J. Biol. Chem. 287, 23995–24003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thein M. C., Winter A. D., Stepek G., McCormack G., Stapleton G., Johnstone I. L., Page A. P. (2009) Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J. Biol. Chem. 284, 17549–17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barski O. A., Tipparaju S. M., Bhatnagar A. (2008) The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab. Rev. 40, 553–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu Q.-Y., Lu C., Li W.-L., Xiang Z.-H., Zhang Z. (2009) Annotation and expression of carboxylesterases in the silkworm, Bombyx mori. BMC Genomics 10, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nguyen G., Delarue F., Burcklé C., Bouzhir L., Giller T., Sraer J. D. (2002) Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frand A. R., Russel S., Ruvkun G. (2005) Functional genomic analysis of C. elegans molting. PLoS Biol. 3, e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denys A., Allain F., Foxwell B., Spik G. (1997) Distribution of cyclophilin B-binding sites in the subsets of human peripheral blood lymphocytes. Immunology 91, 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ewer J. (2005) How the ecdysozoan changed its coat. PLoS Biol. 3, e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riddiford L. M. (2012) How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 179, 477–484 [DOI] [PubMed] [Google Scholar]
- 53. Kuwano E., Takeya R., Eto M. (1985) Synthesis and anti-juvenile hormone activity of 1-substituted-5-[(E)-2, 6-dimethyl-1,5-heptadienyl]imidazoles. Agric. Biol. Chem. 49, 483–486 [Google Scholar]
- 54. Stenvall J., Fierro-González J. C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arnér E. S. J., Persson O. P., Miranda-Vizuete A., Tuck S. (2011) Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 108, 1064–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chaudhari S. S., Arakane Y., Specht C. A., Moussian B., Kramer K. J., Muthukrishnan S., Beeman R. W. (2013) Retroactive maintains cuticle integrity by promoting the trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 9, e1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suzuki Y., Koyama T., Hiruma K., Riddiford L. M., Truman J. W. (2013) A molt timer is involved in the metamorphic molt in Manduca sexta larvae. Proc. Natl. Acad. Sci. U.S.A. 110, 12518–12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corsi A. K. (2006) A biochemist's guide to Caenorhabditis elegans. Anal. Biochem. 359, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martin R. M., Leonhardt H., Cardoso M. C. (2005) DNA labeling in living cells. Cytometry A 67, 45–52 [DOI] [PubMed] [Google Scholar]
- 59. Firstencel H., Butt T. M., Carruthers R. I. (1990) A fluorescence microscopy method for determining the viability of entomophthoralean fungal spores. J. Invertebr. Pathol. 55, 258–264 [Google Scholar]
- 60. He F. J., MacGregor G. A. (2003) Salt, blood pressure and the renin-angiotensin system. J. Renin Angiotensin Aldosterone Syst. 4, 11–16 [DOI] [PubMed] [Google Scholar]
- 61. Noirot C., Quennedey A. (1974) Fine structure of insect epidermal glands. Annu. Rev. Entomol. 19, 61–80 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.