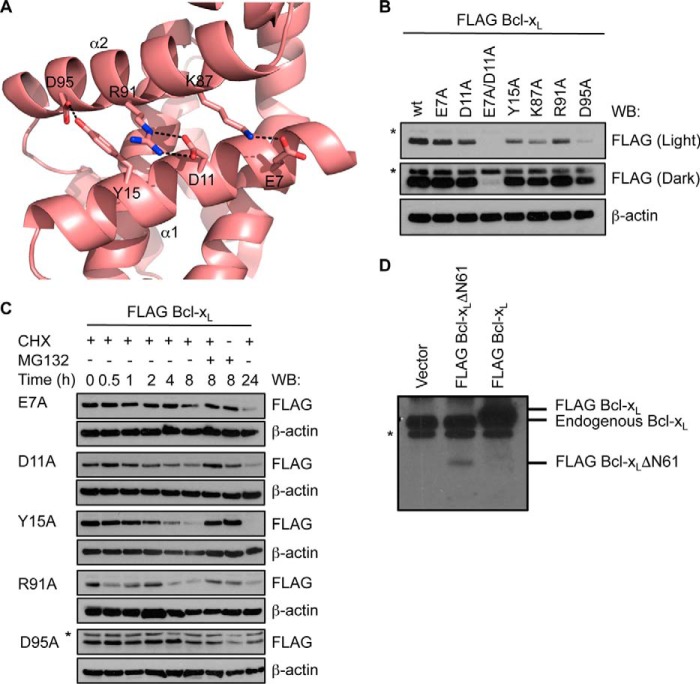
FIGURE 7.
Interactions between α1 and α2 helices stabilize Bcl-xL. A, close-up view of the Bcl-xL crystal structure (PDB code 1PQ0) highlighting the network of interactions between residues on the α1 and α2 helices. B, mutation of residues on α1 or α2 helices impacts steady-state levels of Bcl-xL in MEFs. A long (dark) exposure is provided so that expression of the E7A/D11A mutant can be seen. C, the reduced levels of the mutants are due to their shorter half-life as they are more rapidly degraded by the proteasome following cycloheximide (CHX) treatment. In B and C, Western blots (WB) of equivalent cell lysates were probed with anti-FLAG antibody and then reprobed with anti-β-actin antibody to control for sample loading. The asterisk in C indicates a nonspecific band that becomes apparent due to the longer exposure of this blot. D, Western blot of lysates of cells expressing full-length FLAG-tagged Bcl-xL and FLAG-tagged Bcl-xLΔN61. The asterisk indicates a nonspecific band.