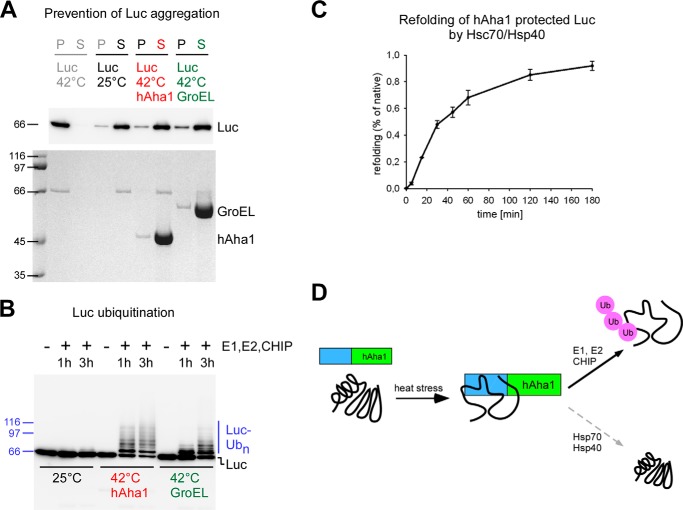
FIGURE 6.
hAha1 prevents aggregation of heat-denatured luciferase and allows client protein ubiquitination by the E3 ubiquitin ligase CHIP. A, hAha1 prevents aggregation of temperature sensitive luciferase (Luc) at 42 °C similar to the chaperonin GroEL. Luciferase remains in the supernatant (S) at 25 °C but precipitates in the pellet (P) at 42 °C in the absence of a chaperone. GroEL and hAha1 are shown on the Coomassie stained SDS-PAGE gel (bottom); the distribution of luciferase between supernatant and pellet is shown on a corresponding Western blot (top). Molecular weights are indicated by marker proteins. B, majority of hAha1 protected luciferase undergoes ubiquitination by the E3 ubiquitin ligase CHIP. Molecular weights are indicated by marker proteins. C, only a minor portion of hAha1-protected luciferase can be reactivated by Hsc70/Hsp40. D, model summarizing the role of the heat stress inducible hAha1 as an autonomous chaperone. hAha1 prevents stress denatured proteins from aggregation and holds them competent for ubiquitination by the E3 ubiquitin ligase CHIP but only to a minor extent for refolding by the Hsc70/Hsp40 chaperone system.