Abstract
Background & objectives:
The presence of CD4+CD8+ (double positive) T cells (DPT) in the target organs of several autoimmune diseases has been reported. The aim of this study was to investigate the pathogenic role of DPT in systemic lupus erythematosus (SLE).
Methods:
A total of 175 SLE cases and 125 matched healthy controls were investigated for CD3+, CD4+, CD8+ lymphocytes and DPT by flow cytometry. Serum samples from SLE patients and controls were tested for antinuclear antibody (ANA), anti-double strain deoxyribonucleic acid (anti-dsDNA), anti-U1 ribonucleoprotein (anti-U1 RNP), anti-sjogren syndrome A (anti-SSA), anti-ribosomal P protein (anti-rib-P), anti-Smith (anti-Sm), anti-Sjogren syndrome B (anti-SSB), complement 3 (C3) and complement 4 (C4).
Results:
The DPT median and 5-95 per cent range of SLE cases and healthy controls were 0.50 [0.10-2.60] and 0.80 [0.20-2.74] respectively (P<0.001). SLE patients were divided into a ≥1:1000 subgroup and a <1:1000 subgroup according to the ANA titre. The DPT of the former subgroup was significantly lower than that of the latter (P=0.032). The DPT medians of positive subgroups with anti-dsDNA (P<0.001), anti-U1RNP (P=0.018), anti-SSA (P=0.021) or anti-rib-P (P=0.039) were also significantly lower than the negative subgroups. Likewise, DPT was significantly lower in SLE subgroups with low concentration of C3 or C4 than those with high concentration (P<0.006).
Interpretation & conclusions:
Our findings show that the DPT cells may play a key suppressive role in the production of autoantibodies in SLE. Direct evidence that DPT regulates the pathogenesis of SLE needs to be investigated in future work.
Keywords: Antinuclear antibody, double positive, pathogenic role, SLE, CD4+CD8+, T cell
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by B cell hyperactivity, abnormal spectrum of autoantibody production and T lymphocyte imbalance1,2,3. In SLE, autoimmune processes attack various tissues and organs by immune complex deposition and lymphocyte infiltration4. CD4+CD8+ double-positive T (DPT) cells represent a sub-population of lymphocytes expressing both CD4 and CD8. It has been reported that DPT cells are present in target organs of several autoimmune conditions, for example, in the thyroid gland of autoimmune thyroiditis, in the skin of atopic dermatitis and systemic sclerosis, and in the joint fluid of rheumatoid arthritis patients5. The presence of DPT cells in target organs suggests their involvement in pathogenic events in most autoimmune diseases6. However, the significance of peripheral DPT cells in the development of SLE has not been reported. In the present study, the peripheral DPT cell expression and the relevant humoral index in SLE were explored retrospectively to define the pathogenic role of DPT in the development of SLE.
Material & Methods
Study design: A case-controlled, single-centre study was carried out between March 2010 and June 2012 in the West China Hospital of Sichuan University, Sichun, PR China, with 175 subjects (19 men, 156 women) with SLE, and 125 (14 men, 111 women) healthy controls with matched age and gender, who were admitted to the Department of Rheumatology or the Medical Examination Center. This study protocol was approved by the Institutional Review Board, and ethics committee of West China Hospital of Sichuan University, PR China. Written informed consent was obtained from all subjects.
Exclusion and inclusion criteria: Patients were included in the SLE group if they satisfied ≥4 of the 11 American College of Rheumatology criteria (1997)7, excluding various malignancies, hepatitis B virus (HBV) or HCV infection, combined other autoimmune disorders, such as rheumatoid arthritis, systemic sclerosis, idiopathic inflammatory myopathies, autoimmune thyroiditis, etc. All healthy controls were volunteers who passed the physical examination, excluding those suffering from autoimmune disorders in their past medical history. All subjects were recruited consecutively according to the inclusion and exclusion criteria for both groups. Blood samples were collected in 3 ml BD vacutainers with heparin anticoagulant for cell analysis and without any anticoagulant for humoral indicators detection. DPT cells and other indicators were analyzed within four hours of collection. Fasting blood samples were used to avoid lipaemic serum, which could result in increased background fluorescence, staining or turbidity. All the experiments in this study were conducted in the Department of Laboratory Medicine, West China Hospital, affiliated to Sichuan University.
Flow cytometry (FCM): Flow cytometry was performed on the BD FACS CANTO™ flow cytometer (Becton Dickinson Corporation, New Jersey, USA). The reagent cocktail (10 μl) containing CD4-flurescein isothiocyanate (FITC), CD8-phycoerythrin (PE) and CD3-peridinin-chlorophyll-protein (PerCP) (Becton Dickinson Corporation, New Jersey, USA) were added to 50 μl heparin anticoagulated whole blood, and the samples were mixed and incubated for 30 min at room temperature. Erythrocytes were lysed by adding 450 μl of ammonium chloride haemolysis agent for 15 min. The lymphocytes were gated as forward-scattered light and side-scattered light, and DPT cells were defined as CD3+CD4+CD8+ T cells by FCM. The histogram of CD4 vs. CD8 showed discrete DPT cell populations (Fig. 1). Subsequent analysis was performed on these cell populations.
Fig. 1.
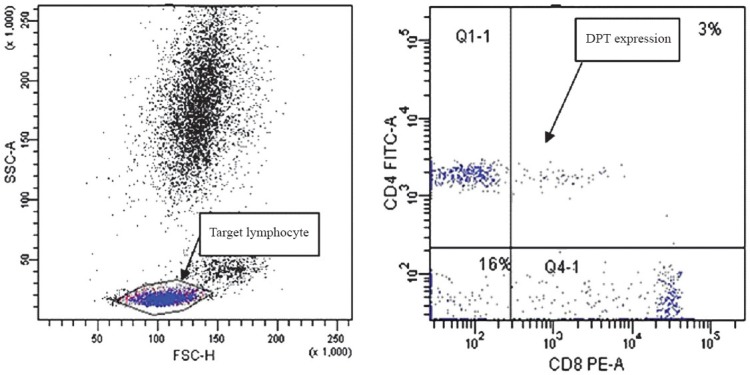
Study of target lymphocytes and double positive T (DPT) cells by flow cytometry (FCM).
Indirect immunofluorescence (IIF): Fresh serum samples of all SLE patients were collected for the detection of antinuclear antibody (ANA) and anti-double strain deoxyribonucleic acid (anti-dsDNA) antibody by IIF. ANA was determined with a commercial ANA kit according to the manufacturer's instructions (EUROIMMUN Corporation, Luebeck, Germany). Serum samples were processed at a dilution of 1:100 using HEp-2/liver biochip (Monkey) and FITC-conjugated anti-human IgG. The fluorescence intensity was scored at ×400 with E6000 fluorescence microscope (Nikon Corporation, Japan). The serum was subsequently diluted to 1:320, 1:1000, 1:3200 and 1:10000 when a positive result was obtained with the lower dilution.
Anti-dsDNA level was analyzed with a commercial anti-dsDNA kit (EUROIMMUN Corporation, Luebeck, Germany). Serum samples were processed at an initial dilution of 1:10 using Crithidia luciliae biochip and FITC-conjugated anti-human IgG. The fluorescence intensity was scored at ×400. The serum was subsequently diluted to 1:32, 1:100, 1:320 and 1:1000 when a positive result was obtained.
Immunoblot assay for extractable nuclear antigen (ENA) antibody profiles: The ENA antibody profiles were tested by line immunoblot assay. The nylon strips contained a plastic backing and were coated with discrete lines of recombinant and purified antigens such as U1RNP, SSA, ribosomal P protein (rib-P) and Sm, along with a control band (EUROIMMUN Corporation, Luebeck, Germany). The nylon strips were incubated with the serum at a 1:100 dilution according to the manufacturer's instructions. The test strips were recorded by the EPSON Expression 1600 scanner (EPSON, Taiwan, China) and analyzed by the Eurolinescan software (EUROIMMUN Corporation, Luebeck, Germany).
Rate nephelometry: Complement 3 (C3) and complement 4 (C4) levels were determined by IMMAGE 800 rate nephelometry with the immunochemistry system (Beckman coulter, California, USA). Each determination was performed as specified in the manufacturer's kit insertion using antiserum, calibrator, buffer solution and diluents provided by the manufacturer.
Statistical analysis: Quantitative data with normal distribution were described by their mean and standard deviation (SD). The non-normal data were described by median and 5~95 per cent range. T-test, Chi-square or non-parametric Mann-Whitney-U test were applied for comparison of groups. All data were analyzed by SPSS 17.0 (IBM, USA).
Results
There were no significant differences between the SLE patients and the controls in terms of the mean age and gender distribution. The mean age of SLE patients was 32.9 ± 12.2 yr and that of controls was 34.2 ± 11.2 yr. Of the 175 SLE patients, 156 (89.1%) were females while in controls 111 of 125 (88.8%) were females.
Characterization of peripheral blood CD3+, CD4+, CD8+ and DPT cells derived from SLE patients and healthy controls: The respective proportions of CD3+ T cells were 70.4 (49.7-86.2) and 63.0 (48.8-79.9); of CD4+ were 29.6 (16.2-45.8) and 32.5 (22.2-45.9); of CD8+ were 34.1 (17.4-58.7) and 23.2 (14.0-39.5); and of DPT cells were 0.50 (0.10-2.60) and 0.80 (0.20-2.74) in SLE patients and controls, respectively. As shown in Fig. 2, the proportions of DPT and CD4+ T cells in SLE patients were significantly lower than those in the controls (P<0.002), while the proportions of CD3+ and CD8+ T cells in SLE patients were significantly higher than those in the controls (P<0.001).
Fig. 2.
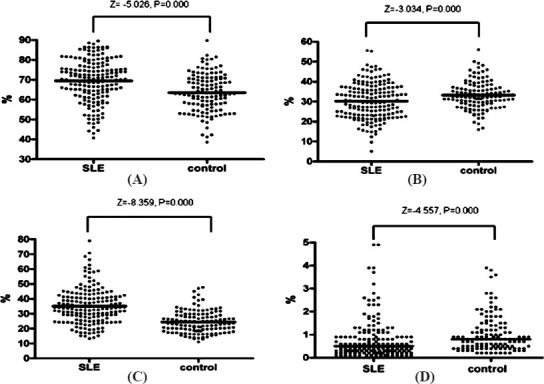
Proportion of T cells in SLE and controls. (A) Proportion of CD3+ T cell subsets in SLE and controls; (B) Proportion of CD4+ T cell subsets in SLE and controls; (C) Proportion of CD8+ T cell subsets in SLE and controls; (D) Proportion of DPT cells subsets in SLE and controls. Note: DPT cells, double positive T (DPT) cells; SLE, systemic lupus erythematosus.
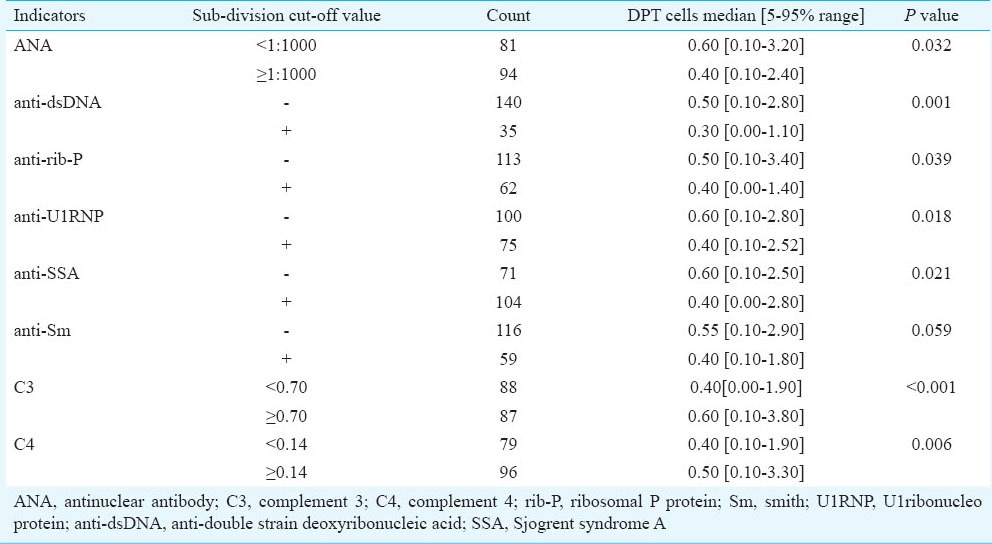
Proportion of DPT cells in different SLE subgroups divided according to other indicators: In our study, none of the healthy controls exhibited autoantibodies (data not shown). However, in the majority of the SLE patients, various autoantibodies were found, such as ANA, anti-dsDNA, anti-U1RNP, anti-SSA, anti-rib-P and anti-Sm. The relationships between CD3+, CD4+, CD8+ and DPT cells and autoantibodies as well as complement concentration were analyzed. No significant and definite relationship was found between CD3+, CD4+, CD8+ T cells and autoantibodies or complement (data not shown). But the proportion of DPT cells was significantly lower in the groups with ANA titre of more than 1:1000, as well as in the positive anti-dsDNA group, the positive anti-rib-P group, the positive anti-U1RNP group, the positive anti-SSA group and the groups with lower concentration of C3 or C4 than the opposite groups (Table I).
Table I.
Proportion of DPT cells in different SLE subgroups
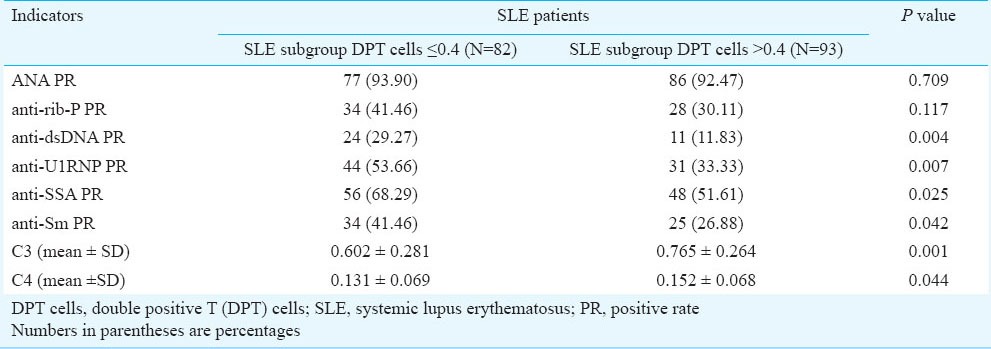
Other indicators in different SLE sub-groups divided according to DPT cells: The SLE patient group was divided into subgroups with lower and higher proportion of DPT cells according to a 0.40 cut-off value, so that the two subdivisions contained a comparable number of patients. The results showed that the production of autoantibodies including anti-dsDNA, anti-U1RNP and anti-SSA was significantly increased in the SLE subgroup with low proportion of DPT cells compared to the SLE subgroup with high proportion of DPT cells. The concentrations of C3 and that of C4, on the other hand, were both lower in the low-DPT group than in the high one (P<0.044), (Table II).
Table II.
Other indicators in SLE subgroups divided according to DPT cells
Discussion
DPT cells were originally thought to be the progenitor of T lymphocytes and to exist in the thymus before maturation8. Blue et al9 in 1986 have reported for the first time that human DPT cells can exist outside of thymus. Low proportions (<3%) of DPT cells increasing with age were found in the peripheral blood of healthy individuals10,11. There were no definitive conclusions concerning the origin and the function of DPT cells. It was reported that DPT cells might derive from immature CD4+CD8+ thymocytes, mature CD4+ single positive (SP) T cells, or mature CD8+ SP T cells5. The DPT cells may also derive from other immune organs, such as the lymph nodes, liver or intestinal tract12. So far, the most convincing hypothesis is that DPT cells are derived directly from CD4 T cells in human peripheral blood, since the characteristic thymocyte marker CD1 is absent. Therefore, these lymphocytes are not immature cells that have escaped from the thymus10. On the other hand, some studies showed that stimulation of human CD4 T lymphocytes with either mitogen or alloantigen in the presence of exogenous interleukin-4 (IL-4) could induce the expression of the CD8α chain13. In vitro studies have provided evidence that CD4 and CD8 molecules expressed on DPT cells can lead to the acquisition of new functions5. DPT cells have the dominant capability of secreting IL-4, IL-13 and assisting B cells with differentiation14,15. In humans, the presence of DPT cells in inflammatory sites16,17, in the lamina propria of the gut and in kidney allografts is suggestive of an immunoregulatory and/or immunosurveillance function. Comparative analyses of the functional differences between CD4+CD8- and CD4+CD8+ DPT will likely provide insight into their roles in cell-mediated immune protection in human graft rejection, immunological tolerance and autoimmune diseases.
It has been demonstrated that DPT cells are capable of mounting a large and highly diversified response to HIV antigens, and represent a major responding population to acute HIV infection18. Research on human melanomas has documented the significantly increased proportion of DPT cells in melanoma tumours compared to blood samples, as well as original cytokine profile11. This indicated that tumour-associated DPT cells could participate in immune responses to tumours in vivo. Studies on nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) also suggested a reactive or regulatory role of DPT in the pathogenesis of this disease19. Results derived from a phenotypic analysis of DPT cells in the blood and liver of patients chronically infected with HCV have suggested that chronic HCV infection promotes the production of DPT cells, perhaps by their re-induction in the thymus and selection in the liver16. Previously studies also found that DPT cells were present in the target organ of several autoimmune conditions, which might suggest their involvement in the pathogenesis of these autoimmune diseases5.
With evidence accumulated for the potential role of DPT cells in many critical illnesses11, no report was found for SLE. In our study, the DPT cells were found to exist in the peripheral blood of both SLE patients and healthy individuals, with the DPT cell proportion in SLE patients significantly lower than that in the controls. This suggested that the DPT cell subset found in the SLE patients could represent a protective T cell subset or that other pathogenic factors in SLE caused the proportion of DPT cells to decrease.
This phenomenon might be explained by the role of cytokines produced by DPT cells. It has been confirmed that IL-6 has the most important effect on inducing the maturation of B lymphocytes into plasma cells and augmenting immunoglobulin secretion20. In human lupus patients, accentuated IL-6 levels correlate with the disease activity and anti-dsDNA levels21. But the production of IL-6 is reduced by IL-4 and IL-1322,23. DPT cells mainly produce IL-4 and IL-13 with a repressive role in the secretion of IL-6. A low proportion of DPT cells means that a high level of IL-6 can be produced, which can promote B lymphocyte proliferation and maturation in SLE, thus leading to an increased autoantibody production. On the other hand, it has been shown that IL-13 contributes to the negative regulation of the immune response23. The decreased proportion of DPT cells in SLE suggests that lower IL-13 level may play less of a role in negatively regulating immune responses including the suppressive role of B cell growth and antibody production. From the analysis it was inferred that the decline of DPT cells would be related to immune activation and might be an important factor leading to a worsening of SLE.
Our results showed that the DPT cell concentration was low in presence of low C3 or C4. It was proposed that more autoantibodies would lead to more immune complexes, which would subsequently activate classic complement pathways, resulting in a decreased complement level. The decreased proportion of DPT cells observed in this study was consistent with the hypothesis that active SLE patients possess more autoantibodies and less C3 and C424.
In conclusion, our data showed that the DPT cell proportion was lower in patients with SLE compared to healthy individuals, especially in the active status of disease. It also indicated that DPT might play a key suppressive role in the production of autoantibodies in SLE. Our study also demonstrated the potential relationship between DPT cells and humoral indicators in SLE. Results imply a negative association between the proportion of DPT cells and the activity of SLE, which suggests a potential role of DPT cells as an indicator of the clinical degree of SLE. Direct evidence that DPT regulates the pathogenesis of SLE needs to be investigated in future studies with a large samples and proper study design.
Acknowledgment
The authors thank Dr Qian Gao and Dr Xinle Liu for data preparation and reviewing the manuscript. This study was supported by the National Natural Science Foundation of China No. 81202354 and Public Benefit Research Project No. 201202004.
References
- 1.Jacob N, Stohl W. Autoantibody-dependent and autoantibody-independent roles for B cells in systemic lupus erythematosus: past, present, and future. Autoimmunity. 2010;43:84–97. doi: 10.3109/08916930903374600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyttaris VC, Tsokos GC. T lymphocytes in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2004;16:548–52. doi: 10.1097/01.bor.0000132646.55056.e0. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh P, Dwivedi S, Naik S, Agarwal V, Verma A, Aggarwal A, et al. Antinuclear antibodies by indirect immunofluorescence : optimum screening dilution for diagnosis of systemic lupus erythematosus. Indian J Med Res. 2007;126:34–8. [PubMed] [Google Scholar]
- 4.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun Rev. 2004;3:215–20. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Rahemtullah A, Harris NL, Dorn ME, Preffer FI, Hasserjian RP. Beyond the lymphocyte predominant cell: CD4+CD8+ (T-cells in nodular lymphocyte predominant Hodgkin lymphoma. Leuk Lymphoma. 2008;49:1870–8. doi: 10.1080/10428190802308728. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun. 2014;48-49:10–3. doi: 10.1016/j.jaut.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Laky K, Fleischacker C, Fowlkes BJ. TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol Rev. 2006;209:274–83. doi: 10.1111/j.0105-2896.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 9.Blue ML, Daley JF, Levine H, Craig KA, Schlossman SF. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986;137:1202–7. [PubMed] [Google Scholar]
- 10.Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72:55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 11.Desfrancois J, Moreau-Aubry A, Vignard V, Godet Y, Khammari A, Dreno B, et al. Double positive CD4CD8 alphabeta T cells: a new tumor-reactive population in human melanomas. PLoS One. 2010;5:e8437. doi: 10.1371/journal.pone.0008437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins C, Norris S, McEntee G, Traynor O, Bruno L, von Boehmer H, et al. RAG1, RAG2 and pre-T cell receptor alpha chain expression by adult human hepatic T cells: evidence for extrathymic T cell maturation. Eur J Immunol. 1996;26:3114–8. doi: 10.1002/eji.1830261243. [DOI] [PubMed] [Google Scholar]
- 13.Brod SA, Purvee M, Benjamin D, Hafler DA. Frequency analysis of CD4+CD8+ T cells cloned with IL-4. Cell Immunol. 1990;125:426–36. doi: 10.1016/0008-8749(90)90096-a. [DOI] [PubMed] [Google Scholar]
- 14.Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci USA. 2003;100:5324–9. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–86. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 16.Nascimbeni M, Pol S, Saunier B. Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. PLoS One. 2011;6:e20145. doi: 10.1371/journal.pone.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frahm MA, Picking RA, Kuruc JD, McGee KS, Gay CL, Eron JJ, et al. CD4+CD8+ T cells represent a significant portion of the anti-HIV T cell response to acute HIV infection. J Immunol. 2012;188:4289–96. doi: 10.4049/jimmunol.1103701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan NK, Vajpayee M, Mojumdar K, Singh R, Singh A. Study of CD4+CD8+ double positive T-lymphocyte and function in Indian patients infected with HIV-1. J Med Virol. 2012;84:845–56. doi: 10.1002/jmv.23289. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee D. Recent advances in the pathobiology of Hodgkin's lymphoma: Potential impact on diagnostic, predictive, and therapeutic strategies. Adv Hematol 2011. 2011 doi: 10.1155/2011/439456. 439456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–43. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18:565–70. [PubMed] [Google Scholar]
- 22.Desfrancois J, Derre L, Corvaisier M, Le Mevel B, Catros V, Jotereau F, et al. Increased frequency of nonconventional double positive CD4CD8 alphabeta T cells in human breast pleural effusions. Int J Cancer. 2009;125:374–80. doi: 10.1002/ijc.24366. [DOI] [PubMed] [Google Scholar]
- 23.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasiri S, Karimifar M, Bonakdar ZS, Salesi M. Correlation of ESR, C3, C4, anti-DNA and lupus activity based on British Isles Lupus Assessment Group Index in patients of rheumatology clinic. Rheumatol Int. 2010;30:1605–9. doi: 10.1007/s00296-009-1201-3. [DOI] [PubMed] [Google Scholar]


