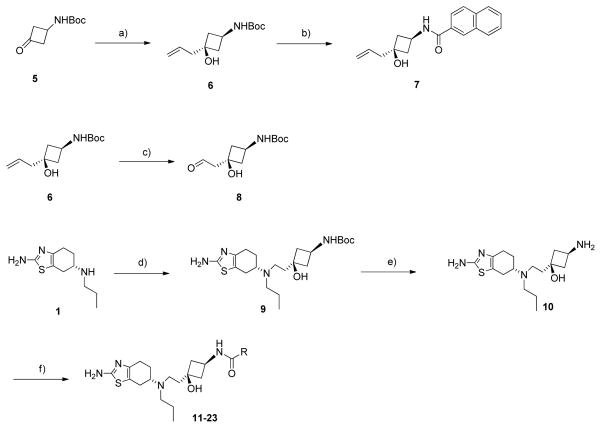
Scheme 1.
Synthesis of compounds 11-23. Conditions and reagents: a) Allylmagnesium bromide, THF, −78 °C, 4 h, 68%; b) 1. TFA, DCM, RT, 12 h, 2. 2-naphthoyl chloride, DIPEA, DCM, RT, 2 h, 72%; c) OsO4, NalO4, THF-H2O, RT, 30 min, 60%; d) 8, NaBH(OAc)3, HOAc, DCM, 27%; e) TFA, DCM, RT, 12 h, 90%; f) appropriate acid chlorides, DIPEA, DCM, RT, 2 h, 36–66%.