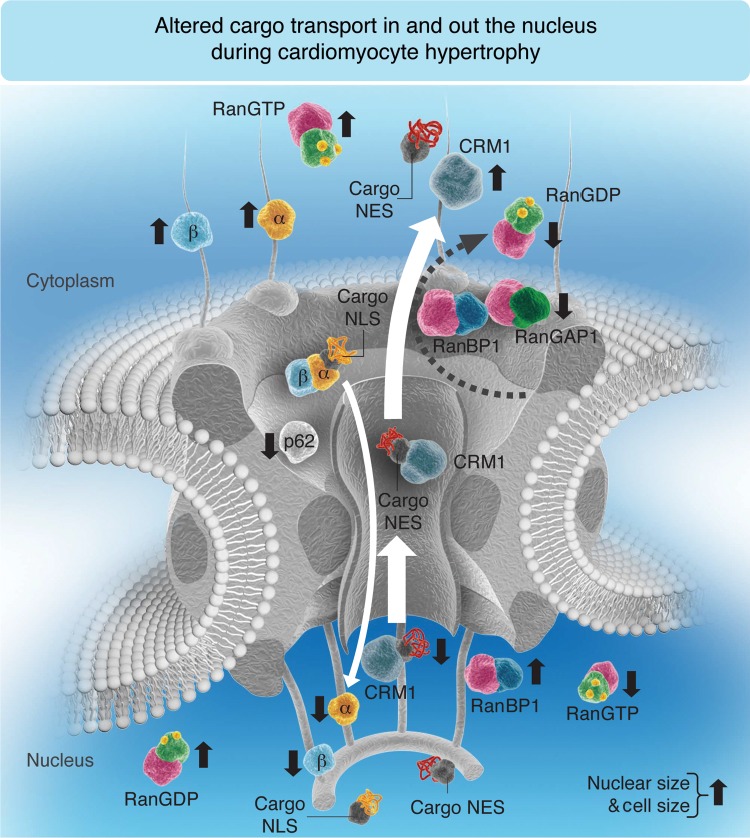
Figure 8.
Hypothetical model occurring in myocardial tissue and isolated CM under hypertrophic conditions. Compared with healthy control (CT) rat CM or healthy human myocardial tissue, the balance between NPI and NPE is disrupted in rat CM exposed to PE for 48 h or with ischaemic cardiomyopathy (rICM) or human cardiac tissue with DCM; under these hypertrophic stimuli, the demand for the nuclear export of transcription factors is exaggerated so that the export receptor (CRM1) is found to be constantly in the cytoplasm. Subsequently, RanBP1, which facilitates the GTP hydrolysis with RanGAP1, cannot be translocated into the cytoplasm by CRM1, and the other import transport receptors (importins-α and -β) are found sequestered in the cytoplasm with RanGTP. Thus, no importins are available in the nucleus to be recycled and released free in the cytoplasm for another import cycle, which can explain the decrease in NPI. In conclusion, under hypertrophic conditions where nuclear and cell sizes are increased, CRM1 cytoplasmic translocation is increased, whereas cytoplasmic RanBP1, Nup p62, and nuclear translocation of importins (α and β) are decreased. The size of the white arrow is proportional to the transport rate. An upward black arrow shows an increase, whereas a downward black arrow shows a decrease. (Note that for simplification, some transport machinery components such as CAS, RanGEF, and NTF2 are not included in this schematic representation since this model is mostly summarizing the elements mentioned in this paper.)