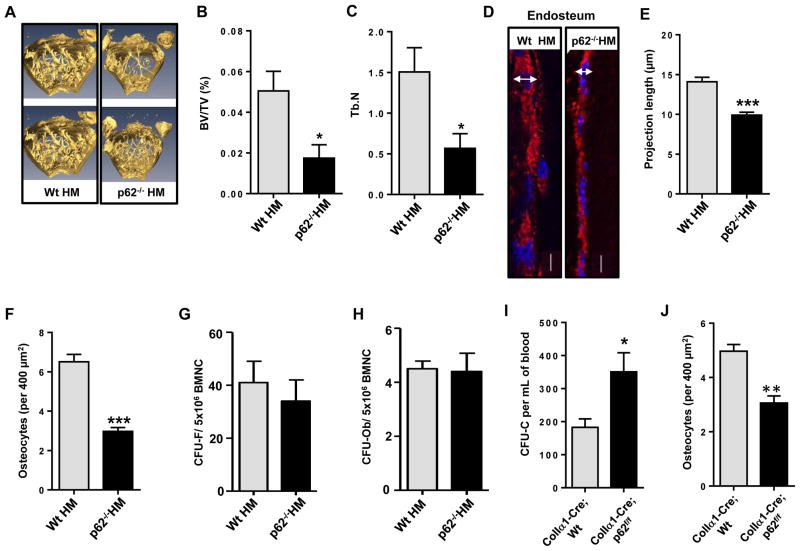
Figure 2. Ob p62-deficiency impairs Ob differentiation and osteogenesis in vivo resulting in HP egress.
(A) Representative micro-computer tomography (micro-CT) analyses of femoral trabecular bone of Wt HM or HM p62−/− mice after 16 weeks of transplantation. (B and C) Percent ratio of trabecular bone volume versus tissue volume (BV/TV) and trabecular number (Tb.N) of Wt HM or p62−/− HM mice (4–7 mice per group) at 16 weeks after transplantation. (D) Representative confocal microscopic images of collagen type1α1 (red) and nuclear counterstaining (DAPI, blue) in femur sections from Wt HM or p62−/− HM. (E) Measurement of Ob length projections of bone lining Obs in longitudinal femoral sections from Wt HM (Ob n=24) or p62−/− HM (Ob n>17 per group). Analysis was performed as measurement of the transversal diameter at the widest point of the Ob expressing Col1α1 (space between arrow ends). (F) Counts of osteocytes in femoral cortical bone from Wt HM (n=47 fields) or p62−/− HM (n=42 fields). Values represent mean ± SEM. ***p<0.001. (G and H) CFU-F and CFU-Ob from BMNC of chimeric Wt HM or HM p62−/− mice. (I) ColIa1-Cre;p62 p62f/f mice mice phenocopy the hematopoietic egress of primary p62−/− mice and p62−/− HM mice. Values represent mean ± SEM. *p<0.05. (J) Osteocyte counts in femoral cortical bone from Coll1α1-Cre; Wt or Coll1α1-Cre; p62f/f. N=33 fields were analyzed for each group. ***p<0.001.