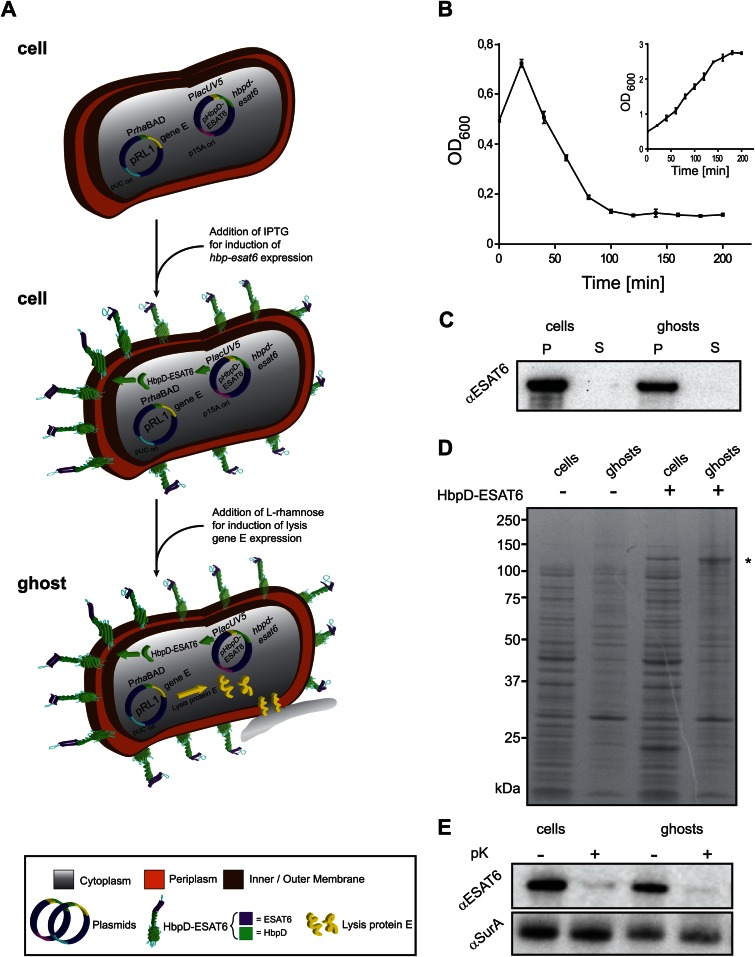
FIG 4.
Hbp-mediated surface display of antigen ESAT6 in E. coli ghosts. (A) A schematic representation of how E. coli-derived ghosts displaying ESAT6 on their surface are generated. MC4100/pRL1 cells are transformed with the with pRL1-compatible plasmid pHbpD(p15A)-ESAT6, which harbors hbpd-esat6 under the control of the lacUV5 promoter. Expression of hbpd-esat6 is induced by the addition of IPTG. Expression of lysis gene E is induced by the addition of l-rhamnose and results in the formation of ghosts. (B) E. coli MC4100 cells harboring pRL1 and pHbpD(p15A)-ESAT6 were cultured in LB at 30°C. Expression of hbpd-esat6 was induced by the addition of 0.4 mM IPTG at an OD600 of 0.25. Expression of lysis gene E was induced with 6 mM l-rhamnose during mid-log phase, at an OD600 of 0.5 (time = 0 min). The optical density (OD600) of the culture was monitored at the indicated time points during a period of time of 200 min. The inset represents a culture of MC4100 cells harboring pRL1 and pHbpD(p15A)-ESAT6 to which no l-rhamnose was added. n = 3; error bars indicate ±SD. (C) The presence of HbpD-ESAT6 in cells and ghosts and the culture media was monitored by SDS-PAGE/immunoblotting using anti-ESAT6. P, pellet; S, supernatant. (D) The abundance of HbpD-ESAT6, marked by an asterisk (*), in cells and ghosts was monitored by SDS-PAGE followed by Coomassie blue staining. As controls, cells and ghosts without any HbpD-ESAT6 were used. (E) Top panel, surface exposure of HbpD-ESAT6 in cells and ghosts was monitored by means of proteinase K accessibility combined with SDS-PAGE/immunoblotting using a monoclonal antibody against ESAT6. Bottom panel, cell envelope integrity during the procedure was demonstrated by showing the inaccessibility of the periplasmic chaperone SurA to proteinase K using anti-SurA.