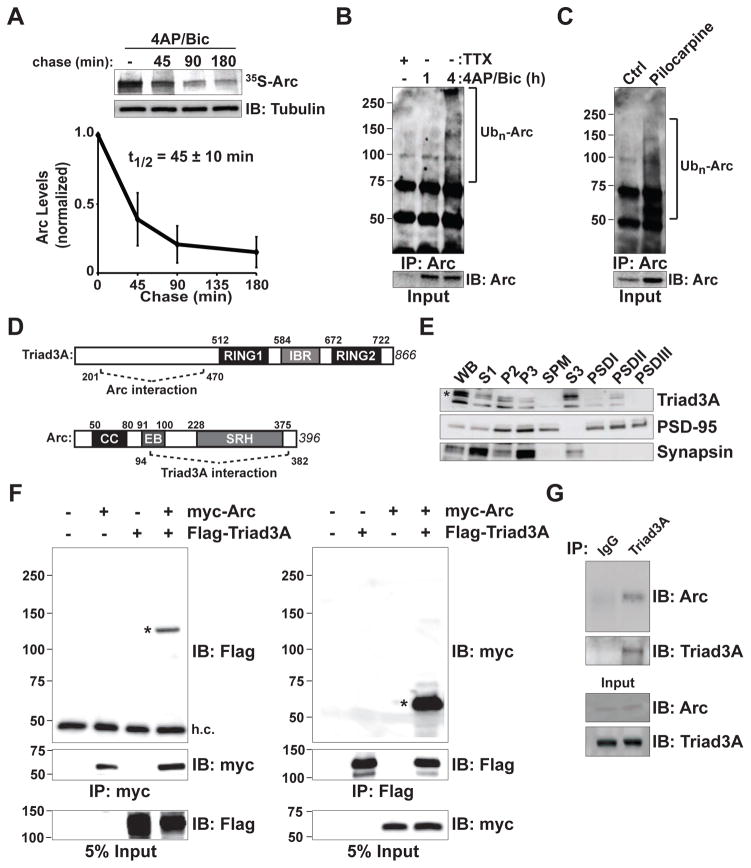
Figure 1. Rapid Turnover of Arc and Interaction with the RING E3 Ligase Triad3A.
(A) Pulse-chase analysis of endogenous Arc. Cortical neurons at 14–18 days in vitro (DIV14–18) were treated with 40 μM 4-aminopyridine (4AP) and 50 μM bicuculline (Bic) for 4 h in the presence of 35S-Cys/Met. Labeled neurons were chased with unlabeled amino acids for various times in the continued presence of 4AP/Bic. Cell lysates were immunoprecipitated (IP) with an anti-Arc antibody, resolved by SDS-PAGE, and visualized by autoradiography. The remaining labeled Arc was plotted over time. Data indicate means ± SEM and half-life (t1/2) is shown. n = 3.
(B) Endogenous Arc is upregulated and ubiquitinated upon 4AP/Bic treatment. DIV14 cortical neurons were incubated with 4AP/Bic for 1 and 4 h. Endogenous Arc was immunoprecipitated (IP) under denaturing conditions, resolved by SDS-PAGE and immunoblotted (IB) with an antiubiquitin (Ub) antibody. Brackets in (B) and (C) indicate polyubiquitinated Arc species.
(C) Arc is ubiquitinated in vivo. Hippocampal extracts were isolated from mice following pilocarpine-induced seizures to elevate Arc levels. Endogenous ubiquitinated Arc was immunoprecipitated and detected as in (B).
(D) Identification of Triad3A as a binding partner of Arc by yeast two-hybrid screening. Schematic diagram indicates domain structures of Arc and Triad3A and their identified binding regions. Numbers indicate amino acid residues. CC, coiled-coil domain; EB, endophilin-3 binding domain; SRH, spectrin repeat homology domain; RING (Really Interesting New Gene) domain; IBR, In-Between-RING domain.
(E) Subcellular distribution of Triad3A in brain. Subcellular fractions from adult rat forebrain were immunoblotted for Triad3A, PSD-95, and synapsin I. For each lane, 25 μg of S1, P2, and P3 fractions, or 8 μg each of total synaptosomal plasma membrane (SPM) and S3 fractions, or 3 μg of postsynaptic density (PSD) fractions (PSDI, PSDII, and PSDIII) were loaded for immunoblot analysis. Note that Triad3A is present in both S3 and PSDII fractions.
(F) Triad3A and Arc form a complex in heterologous cells. FLAG-Triad3A and myc-Arc were expressed in HEK293 cells and immunoprecipitated (IP) with antibodies to their corresponding epitope tags (myc, left; Flag, right), followed by immunoblotting (IB) with anti-Flag (left) or anti-myc antibodies (right). Asterisks represent the corresponding co-immunoprecipitation of Triad3A or Arc. h.c., IgG heavy chain band. Molecular mass markers are shown on the left.
(G) In vivo interaction of endogenous Arc and Triad3A in mouse hippocampus. Following seizure induction with pilocarpine, lysates from mouse hippocampus were immunoprecipitated (IP) with an anti-Triad3A antibody and precipitated proteins were subjected to immunoblot (IB) analysis using an anti-Arc antibody.