Table 1. Reaction Optimizationa.
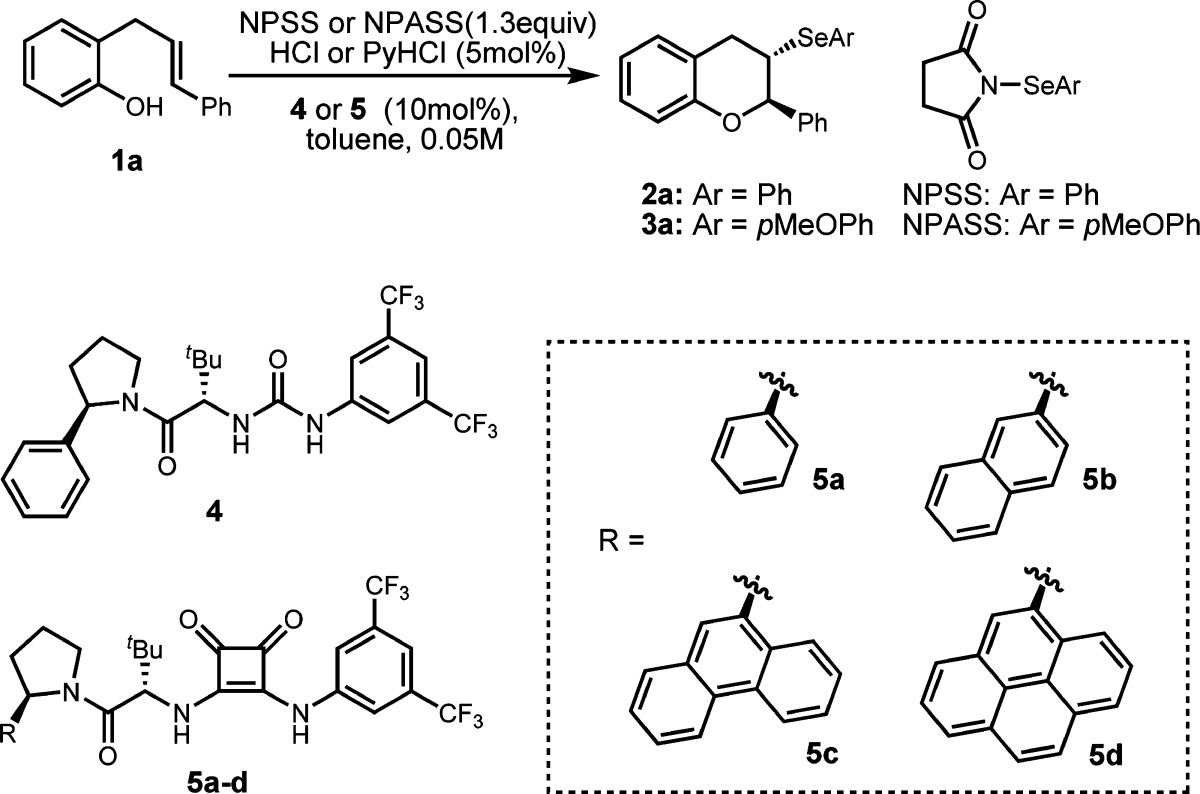
| entry | [Se] | acid | cat. | temp (°C) | time (h) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|---|
| 1 | NPSS | HCl | 4 | rt | 0.5 | 92 | 33(11d) |
| 2 | NPSS | PyHCl | 4 | rt | 2 | 85 | 36(36d) |
| 3 | NPSS | PyHCl | 5a | rt | 2 | 82 | 46 |
| 4 | NPSS | PyHCl | 5b | rt | 2 | 82 | 60 |
| 5 | NPSS | PyHCl | 5c | rt | 2 | 88 | 64 |
| 6 | NPSS | PyHCl | 5d | rt | 2 | 84 | 67 |
| 7 | NPSS | PyHCl | 5d | –30 | 48 | 67 | 72 |
| 8 | NPSS | PyHCl | 5d | –45 | 48 | <5 | nd |
| 9e | NPSS | PyHCl | 5d | –45 | 48 | 89 | 84 |
| 10e | NPASS | PyHCl | 5d | –45 | 48 | 85 | 87 |
| 11e | NPASS | PyHCl | 5d | –35 | 48 | 85 | 88 |
| 12e | NPASS | HCl | 5d | –35 | 48 | 87 | 88 |
Reactions conducted on 0.1 mmol scale.
Isolated yield of purified product.
Enantiomeric excess determined by HPLC analysis on commercial chiral columns.
ee after 12 h.
HMPA(S) (10 mol%) was added.
