Figure 5.
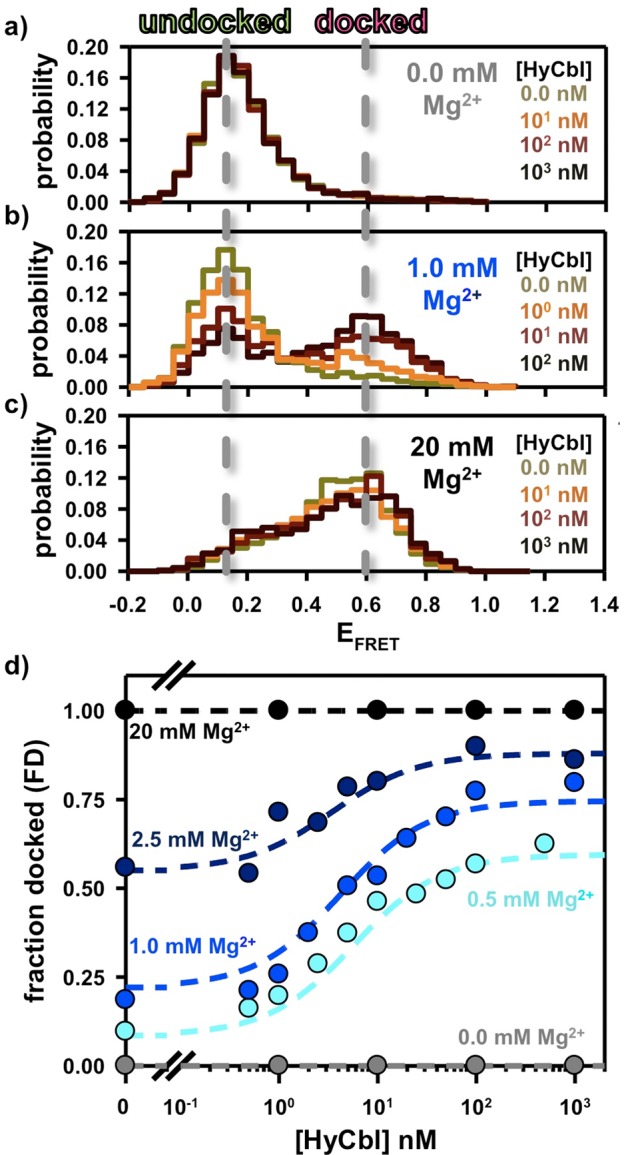
Freely diffusing single-molecule burst titrations of hydroxocobalamin (HyCbl) at various concentrations of MgCl2. (a) At 0 mM Mg2+, addition of ligand does not significantly influence the distribution of EFRET associated with the L5–L13 regulatory switch. (b) At intermediate concentrations of Mg2+(e.g., 2.5 mM), addition of HyCbl does significantly alter the distribution of EFRET. (c) Mg2+ (20 mM) is already sufficient to completely form the L5–L13 KL interaction, irrespective of [HyCbl]. Together, these results support the notion that docking of the env8HyCbl riboswitch is more favorable when the ligand is bound to the RNA under near-physiological salt conditions, but that HyCbl alone (i.e., 0 mM Mg2+) is insufficient to promote formation of the KL interaction. Note the color scheme in a–c is only intended to represent the increasing [HyCbl]; colors do not necessarily correlate one-to-one with [HyCbl]. (d) Experimental validation of the four-state kinetic model for the env8HyCbl riboswitch. The experimentally determined fractional occupancy of the docked conformation (FD, colored circles) is well described by the steady-state solution (dotted line) to the four-state model (Figure 2) over a wide range of Mg2+ and HyCbl concentrations.
