Scheme 5. Kinetic Model for ProcM-Catalyzed Maturation of ProcA2.8.
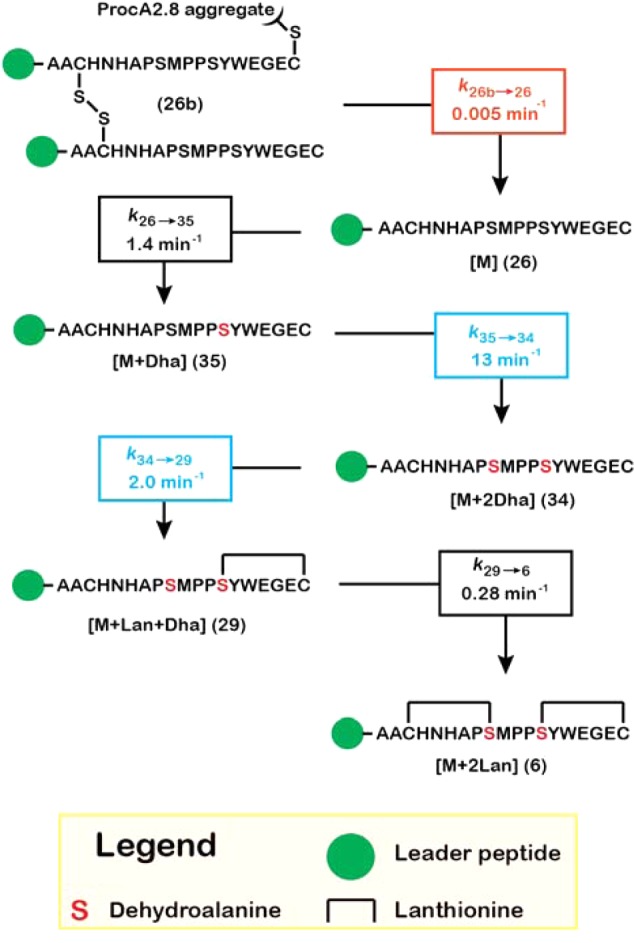
For clarity, peptide binding and dissociation steps from the enzyme are not shown. These steps were included in the model (Scheme 4) and were held constant for each species, as described in the text. Rate constants are color-coded: black, allowed to vary freely during nonlinear regression; red, held fixed at the indicated value; blue, held at a fixed ratio relative to each other. The progress curves simulated for each species in this mechanism are shown along with the experimental data in Figure 5. Compound numbering corresponds to the NEM-alkylated forms of the peptides that were observed in the mass spectra for the reaction (Figure 1D) and are assigned in the Supporting Information (Figure S4, Table S2). Evidence for the putative ProcA2.8 aggregate (26b) is discussed in more detail in the Supporting Information.
