Abstract
Background
Esophagogastroduodenoscopy (EGD) and gastric emptying scintigraphy (GES) are commonly performed in the evaluation of children with upper gastrointestinal (GI) symptoms. It has been presumed, but not clarified, that gastroparesis increases the likelihood of identifying abnormalities on EGD. We sought to determine whether the presence of gastroparesis influenced the diagnostic yield of EGD in children.
Methods
We conducted a retrospective chart review of children who underwent both an EGD and gastric emptying scintigraphy (GES) within 3 months of each other for evaluation of upper GI symptoms (e.g. abdominal pain). Clinical history (symptoms, comorbidities, medications, and surgical procedures), GES results, and EGD histology reports were captured.
Results
125 children (46% female) were included, of whom, 70 (56%) had gastroparesis. 33 (26%) children had liquid-meal GES (1.2 ± 1.1 years of age, mean ± SD) and 92 (64%) had solid-meal GES (12.4 ± 3.6 years of age). There was an overall trend toward a decreased frequency of biopsy abnormalities in those with gastroparesis (P=0.09). Those with gastroparesis identified via liquid-meal GES were less likely to have reflux esophagitis on biopsy (P=0.002). Those with gastroparesis identified on solid-meal GES were less likely to have gastritis (P=0.04). Symptoms, comorbidities, or medications were not predictive of GES or EGD results.
Conclusions
Children with gastroparesis may be less likely to have biopsy abnormalities identified on EGD in comparison to those without gastroparesis. Further prospective, larger, and multicenter studies are needed to validate our findings.
INTRODUCTION
Gastroparesis is a gastrointestinal (GI) motor disorder in which the emptying of the stomach is abnormally delayed in the absence of an anatomic obstruction1. The symptoms of gastroparesis are non-specific and may include abdominal discomfort/pain, nausea, vomiting, fullness/bloating and early satiety. These symptoms, can also be found in patients with other GI disorders (e.g., peptic disease, eosinophilic gastritis).1, 2 Given this symptom overlap among disorders, children may undergo extensive evaluation to determine a specific diagnosis.
Gastric emptying scintigraphy (GES), involving nuclear tagging of an ingested meal, is the current gold standard for diagnosing gastroparesis.3 While GES is a reliable tool for capturing delayed gastric emptying, it may not delineate which GI symptoms are due to the delayed emptying. Further, adult studies have produced mixed results and have not been able to consistently demonstrate a direct correlation between GES results and specific GI symptoms.4, 5 As a result, medical management of gastroparesis with prokinetics and/or diet modification may not resolve all of a patient’s symptoms, prompting further diagnostic work-up.
While GES and other imaging studies are used to evaluate for functional and anatomic abnormalities, respectively, Esophagogastroduodenoscopy (EGD) primarily evaluates for mucosal disease. EGD is commonly employed when a specific GI disease is suspected (e.g., H. pylori, celiac disease, etc.), but may also be used in the evaluation of nonspecific upper GI symptoms.6 To this end, not only may EGD be utilized in children with suspected gastroparesis to ensure an identifiable underlying etiology is not playing a role, but it may be employed in evaluation of children with known gastroparesis who have persistent symptoms despite therapy.7 Data to support the endoscopic evaluation of persistent upper GI symptoms in children with known gastroparesis are lacking, as the diagnostic yield of EGD in this population has not been studied. Therefore, we sought to determine the diagnostic yield of EGD with gastroparesis as compared to those without gastroparesis in children with similar clinical symptoms. We hypothesized that children with normal gastric emptying were likely to have fewer histologic findings than those with gastroparesis.
MATERIALS AND METHODS
Study Design
We conducted a retrospective chart review on 476 children ranging from 1 month - 18 years of age who underwent a GES study at Texas Children’s Hospital over an 8-year period (2003-2010). Of these subjects, we included those who had an EGD performed within 3 months of (before or after) the GES as a part of their evaluation of upper GI symptoms. These evaluations were carried out at the discretion of the 20 various members of the Pediatric Gastroenterology, Hepatology, and Nutrition section who see more than 16,000 outpatients annually, who are referred from primary care physicians. Children with a known history of pyloric surgery, bowel resection, hiatal hernia repair, inflammatory bowel disease, or celiac disease were excluded due to the potential confounding effect these factors might have on GES results.
During the reviewed period, GES studies were conducted over 90 minutes. For liquid studies, the formula the child normally was fed was utilized. Scrambled eggs (120 mL) were used for solid studies. Results were recorded as half-emptying time (T1/2), as calculated by the linear fit method. Gastroparesis was defined as a T1/2 > 60 minutes for a liquid meal, and > 90 minutes for a solid meal based on our institution’s pre-determined standards.8-10 Patients 5 years of age and older who underwent a liquid GES were excluded, as were children under 4 years of age who underwent a solid GES, as there were few subjects in these groups and thus, they were outliers from the general population who underwent GES. Subjects who vomited during their GES were excluded. To avoid repeat data from subjects with multiple GES studies, only the first GES performed was included in the study.
The study protocol was approved by Baylor College of Medicine’s institutional review board.
Medical Record Assessments
Manual chart reviews of all medical records prior to GES studies with systematic capture of relevant data were carried out to determine GI symptoms. These data included outpatient, inpatient, and telephone notes that pertained to the subject’s GI evaluation. Symptoms captured included abdominal pain, nausea, vomiting, difficulties finishing a meal, constipation, and weight loss. Patients who had a history of early satiety, poor/decreased appetite, or who were eating less than usual were defined as having “difficulties finishing a normal meal.” A weight loss of ≥ 10% was considered significant. Medication use prior to both EGD and GES was recorded in a qualitative manner, and therefore dosage and duration of use were not captured. Medication use was defined as any documentation of its active use in the progress or telephone notes prior to the procedure/study.
Histological Assessment
EGD histological findings were reviewed. If multiple endoscopies were performed during this time, histological results from the EGD performed closest to the date of the GES were used. Evidence of diffuse inflammation with architectural or glandular distortion was required for a diagnosis of histologically significant esophagitis, gastritis, or duodenitis. Nonspecific histological findings (reactive changes, edema, or mild inflammatory changes) were not considered significant for the purpose of this study. For a histological diagnosis of eosinophilic esophagitis, gastritis, or duodenitis, eosinophil counts of at least 15, 30, and 20 per high-powered field, respectively were required.11
Statistical Analysis
Chi-square analysis was used to compare categorical data between groups. IBM Statistical Package for the Social Sciences (Armonk, New York) version 19 software was used for all statistical analysis. Unless otherwise specified, data are presented as mean ± standard deviation.
RESULTS
During the 8-year study period, 476 GES studies were reviewed. 125 children (46.4% female) met the inclusion criteria of having an EGD performed within 3 months of GES as a part of their GI evaluation of upper GI symptoms. The average age of the total cohort was 9.4 ± 5.8 years, while the average age for those who underwent liquid versus solid meal GES was 1.2 ± 1.1 vs 12.4 ± 3.6 years, respectively. The total population was composed of the following races/ethnicities: Caucasian (60%), African-American (8%), Latino (16.8%), Asian (5.6%), and 9.6% of unknown or undocumented ethnicity (Table 1). Gastroparesis was identified in 70/125 (56%). Children with gastroparesis did not differ from those with normal gastric emptying with respect to age, gender, comorbidities, GI symptoms, or medications prior to EGD (Tables 1 & 2).
| Normal Gastric Emptying (n = 55) |
Gastroparesis (n = 70) |
P | |
|---|---|---|---|
| Sex [n (%)] | Female: 21 (38) | Female: 37 (53) | 0.10 |
| Age (y) | 9.9 ± 5.5 | 9.1 ± 6.1 | 0.45 |
| Ethnicity | |||
| White | 36 | 39 | |
| African | 4 | 6 | |
| American | |||
| Hispanic | 6 | 15 | |
| Asian | 5 | 2 | |
| Unknown | 4 | 8 |
| All GES Studies (N = 125) |
Liquid GES Studies (N = 33) |
Solid GES Studies (N = 92) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
Normal
(N = 55) |
Gastroparesis
(N = 70) |
P |
Normal
(N = 12) |
Gastroparesis
(N = 21) |
P |
Normal
(N = 43) |
Gastroparesis
(N = 49) |
P | |
| Sex | 21 F (38%) | 37 F (53%) | 0.102 | 6 F (50%) | 13 F (62%) | 0.506 | 15 F (35%) | 24 F (49%) | 0.172 |
| Age (y) | 9.9 ± 5.5 | 9.1 ± 6.1 | 0.451 | 1.6 ± 1.4 | 1.0 ± 0.8 | 0.166 | 12.2 ± 3.7 | 12.5 ± 3.5 | 0.657 |
| Surgeries | |||||||||
| Appendectomy | 0 | 2 | 0.206 | 0 | 0 | NS | 0 | 2 | 0.180 |
| Cholecystectomy | 0 | 3 | 0.120 | 0 | 0 | NS | 0 | 3 | 0.099 |
| Cardiac surgery | 2 | 0 | 0.108 | 2 | 0 | 0.54 | 0 | 0 | NS |
| Fundoplication | 6 | 1 | 0.022* | 1 | 1 | 0.679 | 5 | 0 | 0.014* |
| G-tube | 6 | 1 | 0.022* | 2 | 1 | 0.252 | 4 | 0 | 0.029* |
| Comorbidities | |||||||||
| GER | 27 | 26 | 0.180 | 12 | 9 | 0.001 | 15 | 17 | 0.985 |
| Neurodevelopmental delay |
4 | 4 | 0.724 | 3 | 4 | 0.687 | 1 | 0 | 0.283 |
| Genetic disorder | 1 | 2 | 0.706 | 1 | 1 | 0.679 | 0 | 1 | 0.346 |
| Prematurity | 7 | 5 | 0.293 | 6 | 5 | 0.125 | 1 | 0 | 0.283 |
| Connective tissue disease |
0 | 1 | 0.373 | 0 | 0 | NS | 0 | 1 | 0.346 |
| Type 1 diabetes | 2 | 0 | 0.108 | 0 | 0 | NS | 2 | 0 | 0.127 |
| Hypothyroidism | 0 | 1 | 0.373 | 0 | 0 | NS | 0 | 1 | 0.346 |
| Cystic Fibrosis | 2 | 0 | 0.108 | 0 | 0 | NS | 2 | 0 | 0.127 |
| Other† | 0 | 0 | NS | 0 | 0 | NS | 0 | 0 | NS |
| Symptoms | |||||||||
| Abdominal Pain | 33 | 46 | 0.511 | 1 | 3 | 0.614 | 32 | 43 | 0.100 |
| Nausea | 16 | 23 | 0.652 | 0 | 1 | 0.443 | 16 | 22 | 0.455 |
| Vomiting | 35 | 46 | 0.809 | 9 | 18 | 0.443 | 26 | 28 | 0.747 |
| Difficulty w/meals | 22 | 36 | 0.203 | 7 | 14 | 0.632 | 15 | 22 | 0.328 |
| Constipation | 5 | 15 | 0.062 | 0 | 6 | 0.041 | 5 | 9 | 0.369 |
| Weight loss | 3 | 11 | 0.071 | 0 | 0 | NS | 3 | 11 | 0.039 |
| Medications before EGD | |||||||||
| Proton pump inhibitor |
33 | 45 | 0.623 | 7 | 9 | 0.392 | 26 | 36 | 0.184 |
| H2 antagonist | 4 | 2 | 0.252 | 2 | 1 | 0.252 | 2 | 1 | 0.482 |
Fisher exact test < 0.05.
Other = mitochondral disease, metabolic disease, biliary dyskinesia, myopathy, and milk protein allergy.
Bold values indicate statistically significant.
EGD indicates esophagogastroduodenoscopy; GES, gastric emptying scintigraphy.
Whole Population
Children with a gastrostomy tube or fundoplication were less likely to have gastroparesis (P=0.029 and P=0.014, respectively). Children with weight loss were more likely to have gastroparesis (P=0.039). There was no difference between the normal gastric emptying group and the gastroparesis group in regard to age or sex.
For the group as a whole there was a trend (P=0.085) for children with normal gastric emptying (23/55) to have a higher proportion of abnormal histological findings on EGD than those with gastroparesis (19/70) (Figure A). Gastritis was identified more frequently in those with normal gastric emptying (P=0.022; Figure A). Reflux esophagitis was the most common finding (n=31) amongst all subjects but the frequency did not differ between the normal emptying and gastroparesis groups. Other findings, including eosinophilic esophagitis (n=4), duodenitis (n=2), eosinophilic duodenitis (n=2), and H. pylori (n=2) were infrequently found within the total population (Figure A). Of note, there were no differences in antisecretory (e.g., proton pump inhibitor therapy) usage prior to EGD between those with and without gastroparesis (Table 2).
Liquid Meal GES
In children who underwent a liquid meal GES (n=33), those with normal gastric emptying had a significantly higher frequency (7/12) of abnormal histological findings compared to those with gastroparesis (3/21; P=0.008; Figure B). The increased frequency of abnormal histologic findings was due primarily to a greater frequency of reflux esophagitis in the normal gastric emptying group (6/12) compared with the gastroparesis group (1/21, P=0.002; Figure B). Other histologic findings were infrequent and their frequency did not differ between groups (Figure B).
The increased frequency of reflux esophagitis was accompanied by an increased frequency of clinical gastroesophageal reflux in the normal emptying group (12/12) compared with the gastroparesis group (9/21, P=0.001). In contrast, those with normal gastric emptying were less likely to have constipation (0/12) compared to those with gastroparesis (6/21, P=0.041). There was no difference between the normal gastric emptying group and the gastroparesis group in regard to age or sex.
Solid Meal GES
In children who underwent a solid meal GES (n=92), the overall frequency of abnormal histologic findings did not differ between the two groups (16/43 vs 16/49; normal emptying vs gastroparesis, respectively; P=0.647). However, the frequency of histologic gastritis was greater in the normal gastric emptying group (6/43) compared with the gastroparesis group (1/49, P=0.032; Figure C). The frequency of other histologic findings did not differ between groups (Figure C).
Impact of Study Order
EGD was performed first in 59 subjects and GES first in 66. The frequency of having an abnormal finding on the subsequent test did not appear to depend on whether the initial test was normal vs abnormal. In the group in whom EGD was performed first, gastroparesis was found in 24/41 who had a normal EGD vs 7/18 who had an abnormal EGD (P=0.16, Figure 2A). In the group in whom GES was performed first, an abnormal EGD was found in 12/27 who had a normal GES vs 12/39 who had gastroparesis (P=0.26, Figure 2B).
Figure 2.
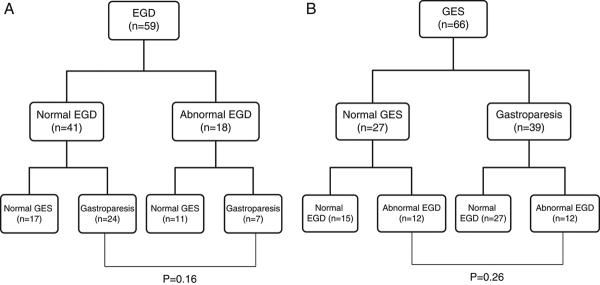
Impact of Study Order. EGD = Esophagogastroduodenoscopy, GES = Gastric Emptying Scintigraphy.
(A) EGD performed prior to GES: Patients with abnormal EGD findings did not differ from those with normal EGD findings in regard to the frequency of subsequent GES findings (P=0.16).
(B) GES performed prior to EGD: Patients with gastroparesis did not differ from those with normal gastric emptying in regard to the frequency of subsequent EGD abnormal findings (P=0.26).
DISCUSSION
To our knowledge, this is the first study to describe the comparative yield of EGD in children with normal gastric emptying versus those with gastroparesis. Contrary to our expectation, we found that children with normal gastric emptying were more likely to have abnormal upper GI histologic findings on EGD compared with those with gastroparesis. Young children who had normal gastric emptying on liquid meal GES were more likely to have esophagitis than comparably aged children with gastroparesis. Older children with normal gastric emptying on solid meal GES were more likely to have gastritis compared with comparably aged children with gastroparesis.
Though potential identifiable etiologies of gastroparesis in children have been reported, our data suggest that for the vast majority of children with upper GI symptoms, having gastroparesis is not predictive of identifying an increase in abnormal histological findings via EGD.7 This may be reflective of the idiopathic nature of gastroparesis in the majority of children.
The frequency with which we found significant histologic findings during EGD was in line with other studies investigating the yield of EGD in other childhood GI disorders, including the report of Thakkar et al. who retrospectively examined the frequency of positive EGD findings in children with recurrent abdominal pain.12 Moreover, clinical characteristics did not significantly differ between those with and without gastroparesis in our study. Thus, although we used retrospective means to identify the two groups, the normal gastric emptying group appeared to be a reasonable comparator to the gastroparesis group.
Reflux esophagitis was the most frequent histologic finding in the overall population (Figure 1A). The most striking frequency (50%) was found in the young children with normal gastric emptying (Figure 1B). This came as somewhat of a surprise given that reflux esophagitis is a known complication of gastroparesis.13 Given the younger age of those who had a liquid vs solid meal GES and the increased frequency of reflux in younger versus older children, it is not surprising that reflux esophagitis was found more frequently in the young children who underwent a liquid GES. However, the exact reason for the difference in esophagitis frequency in young children between the normal emptying and gastroparesis groups remains unclear (Figure 1B). We speculate that the mechanism(s) for causing reflux esophagitis in this age group override any effect that delayed gastric emptying might have in inducing esophagitis. It should be noted that acid suppression medication usage did not differ between groups with/without gastroparesis, or between those with/without specific histological findings.
Figure 1.
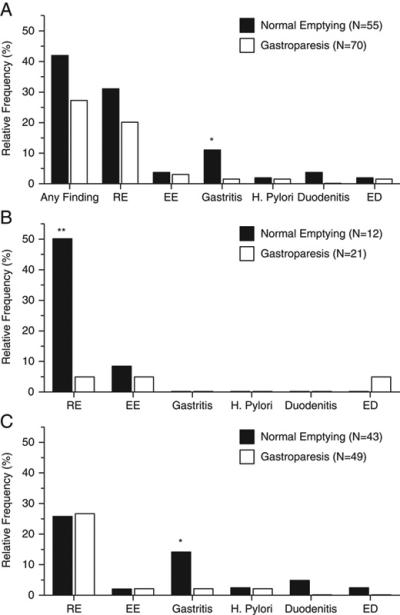
Relative Frequency of Abnormal Histological Findings in Subjects with Normal Gastric Emptying vs Gastroparesis. GER = Gastroesophageal Reflux, EE = Eosinophilic Esophagitis, ED = Eosinophilic Duodenitis.
(A) All GES studies: Gastritis was found more frequently on biopsy in children with normal gastric emptying than in those with delayed gastric emptying (* P < 0.05). Other histological findings did not differ between groups.
(B) Liquid meal GES studies: Reflux esophagitis on biopsy was detected more frequently in children with normal gastric emptying (** P = 0.005). Other histological findings did not differ between groups.
(C) Solid meal GES studies: Gastritis was found more frequently on biopsy in children with normal gastric emptying than in those with delayed gastric emptying (* P < 0.05). This likely accounts for the difference in gastritis frequency for the overall group (Figure A). Other histological findings did not differ between groups.
In the older children, gastritis on biopsy was found more commonly in those with normal gastric emptying compared with those with gastroparesis (Figure 1C). Machado et al., using the 13C-octanoic acid breath test to measure gastric emptying, found that emptying was faster in children with histologic gastritis secondary to H. pylori compared to children without the organism, 80% of whom had normal histology.14 Similar findings were reported by Sýkora and colleagues.15 Whether this is unique to H. pylori gastritis is not clear. However, Friesen et al. found that chronic gastritis in children was not associated with an abnormal solid GES.16 Only 2 of 92 children in our study had H. pylori.
It should be noted that the two most common histological findings amongst all subjects in our study (reflux esophagitis and gastritis) were entities that often respond to acid suppression therapy and/or conservative management. Our results possibly suggest that children presenting with nonspecific upper GI symptoms (abdominal pain, nausea, etc) may benefit from empiric acid suppression therapy prior to performance of an EGD or GES. Approximately 60% of children in our study were so treated. This speculation awaits prospective study.
The main strength of this study is the large number of children studied who were managed during routine clinical practice by a diverse group of 20 pediatric gastroenterologists over many years, none of whom was responsible for the care of a predominance of the subjects. The evaluation of dyspepsia in children is not per protocol at our institution, and therefore the findings are likely to be generalizable and reflect the experience of those caring for children with chronic upper GI symptoms (at least in tertiary care). Another strength of this study is the use of a case vs control comparison. By having those who underwent GES but were found not have gastroparesis as a comparison group, we were able to make more robust observations regarding the frequency of testing in children with gastroparesis and the yield of EGD in this population. The two groups were similar clinically, with the GES result being the primary differentiator.
However, some potential limitations need to be acknowledged. First, the study was retrospective. As such, documentation and clinical decision making were not uniform. However, this limitation may have been ameliorated, in part, by the use of objective measures including the analysis of GES and histological results, thus reducing the risk of bias. Second, generalizability may be limited as there is no current consensus protocol for performing GES in children. However, the same protocol used in our institution has been used in several others.8,10 Nevertheless, the study was conducted at a single center, and therefore may not be generalizable to all centers.
In summary, our data suggest that histologic abnormalities on EGD are more likely to be found in children with normal gastric emptying as opposed to those with gastroparesis. Our findings regarding the relationships between reflux esophagitis and gastritis and gastric emptying in children requires further study. As such, further prospective, larger, and perhaps multicenter studies are needed to validate our findings.
ACKNOWLEDGEMENTS
All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. Gregory Wong is the acting submission guarantor.
FINANCIAL SUPPORT
This study was supported in part by R01 NR05337 (RJS) from the National Institutes of Health, the Daffy’s Foundation (RJS), the USDA/ARS (RJS) under Cooperative Agreement No. 6250-51000-043, and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Funding was also provided by the NASPGHAN Foundation/Nestle Nutrition Young Investigator Development Award (BPC).
Footnotes
All contributing authors do not have any conflicts of interest to disclose.
REFERENCES
- 1.Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat. Rev. Gastroenterol. Hepatol. 2011;8:438–453. doi: 10.1038/nrgastro.2011.116. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 3.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008 Mar;103(3):753–63. doi: 10.1111/j.1572-0241.2007.01636.x. Epub 2007 Nov 19. [DOI] [PubMed] [Google Scholar]
- 4.Cassilly DW, Wang YR, Friedenberg FK, et al. Symptoms of gastroparesis: use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion. 2008;78(2-3):144–51. doi: 10.1159/000175836. Epub 2008 Nov 22. [DOI] [PubMed] [Google Scholar]
- 5.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011 Jul;9(7):567–76. doi: 10.1016/j.cgh.2011.03.003. e1-4. Epub 2011 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelimsky G, Czinn SJ. Techniques for the evaluation of dyspepsia in children. J Clin Gastroenterol. 2001 Jul;33(1):11–3. doi: 10.1097/00004836-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 7.ASGE Standards of Practice Committee. Fukami N, Anderson MA, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc. 2011 Jul;74(1):13–21. doi: 10.1016/j.gie.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Klingensmith WC, 3rd, Lawrence SP. The gastric emptying study: protocol design considerations. J Nucl Med Technol. 2008 Dec;36(4):195–9. doi: 10.2967/jnmt.108.054817. Epub 2008 Nov 13. [DOI] [PubMed] [Google Scholar]
- 9.Weinman J, Gritter K. Gastric emptying: normal values for a simple solid meal [abstract] J Nucl Med. 2006;47:335P. suppl. [Google Scholar]
- 10.Larson JM, Tavakkoli A, Drane WE, et al. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil. 2010 Oct;16(4):407–13. doi: 10.5056/jnm.2010.16.4.407. Epub 2010 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurrell J, Genta R, Melton S. Histopathologic Diagnosis of Eosinophilic Conditions in the Gastrointestinal Tract. Adv Anat Pathol. 2011;18:335–348. doi: 10.1097/PAP.0b013e318229bfe2. [DOI] [PubMed] [Google Scholar]
- 12.Thakkar K, Chen L, Tatevian N, et al. Diagnostic yield of oesophagogastroduodenoscopy in children with abdominal pain. Alimentary Pharmacology and Therapeutics. 2009;30:662–669. doi: 10.1111/j.1365-2036.2009.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waseem S, Islam S, Lahn G, et al. Spectrum of gastroparesis in children. JPGN. 2012 Feb 6; doi: 10.1097/MPG.0b013e31824cf06e. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Machado RS, Reber M, Patrício FR, et al. Gastric emptying of solids is slower in functional dyspepsia unrelated to Helicobacter pylori infection in female children and teenagers. J Pediatr Gastroenterol Nutr. 2008 Apr;46(4):403–8. doi: 10.1097/MPG.0b013e318159224e. [DOI] [PubMed] [Google Scholar]
- 15.Sýkora J, Malán A, Záhlava J, et al. Gastric emptying of solids in children with H. pylori-positive and H. pylori-negative non-ulcer dyspepsia. J Pediatr Gastroenterol Nutr. 2004;39:246–52. doi: 10.1097/00005176-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Friesen CA, Lin Z, Garola R, et al. Chronic gastritis is not associated with gastric dysrhythmia or delayed solid emptying in children with dyspepsia. Dig Dis Sci. 2005;50:1012–1018. doi: 10.1007/s10620-005-2696-4. [DOI] [PubMed] [Google Scholar]


