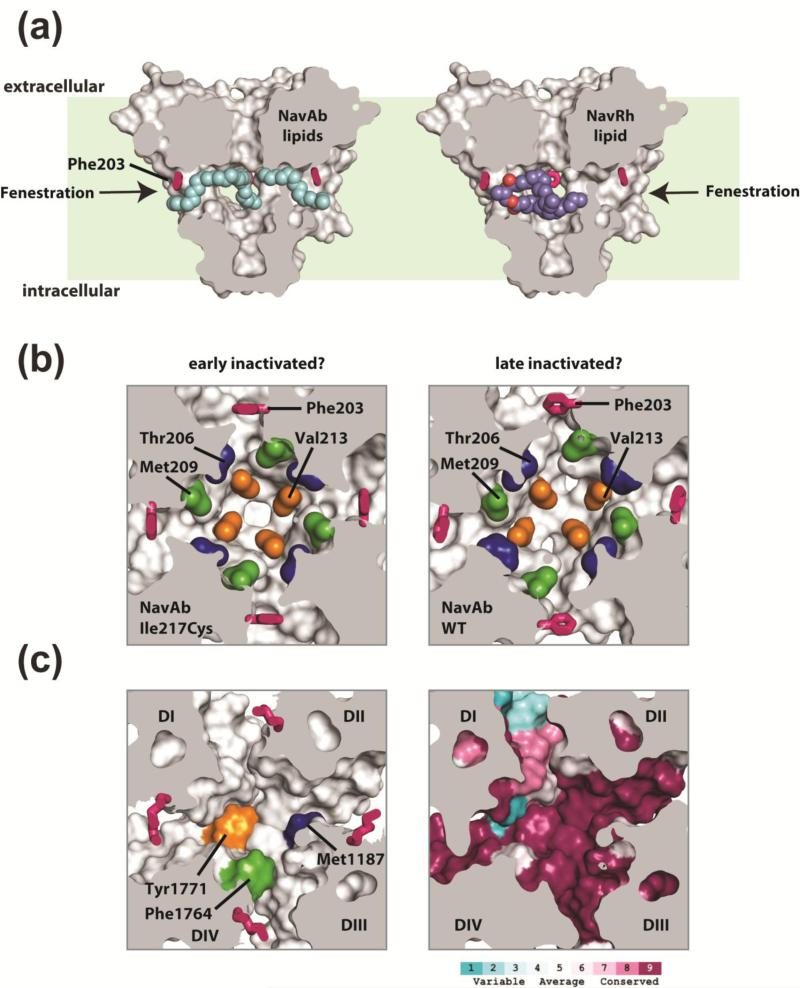
Figure 4. BacNaV PD fenestrations and pharmacology.
(a) NaVAb PD 37 is shown in surface representation sectioned through the middle. Exemplar side fenestrations are indicated by the arrows. The fenestration “gating” residue (Phe203) is shown as pink sticks. Lipids bound within the central cavity of NaVAb are shown as cyan spheres and are seen penetrating through the pore fenestrations (arrows). For easy comparison, the NaVRh PD (PDB 4DXW 39, not shown) was superimposed onto NaVAb and the bound lipid within the NaVRh pore is shown as purple and red spheres. Light green background indicates approximate bilayer boundaries. (b) A sectioned view of NaVAb I217C 37 (left) and NaVAb WT 38 (right) looking into the central cavity, viewed from below the selectivity filter. Phe203 is shown as pink sticks and select sidechains implicated in drug binding and block in eukaryotic NaV and CaV channels are in space filling color (blue, green and orange). The asymmetric central cavity seen in NaVAb WT (right) has been suggested to represent a slow inactivated conformation of the pore, where a reshaping of the pore fenestrations and putative drug binding sites are seen. (c) Homology model of human NaV1.7 based on the NaVAb (PDB 3RVY). Left: select residues implicated in local anesthetic block in DIV S6 of NaV channels (Phe1764 green and Tyr1771 orange) and a residue implicated in DHP block in DIII S6 of rat CaV1.2 (accession: P22002; Met1187 blue) illustrate a potential composite drug receptor site within the central cavity of eukaryotic NaV and CaV channels. Right: sequence conservation analysis for all human NaV channels (NaV1.1- NaV1.9) is mapped onto the NaV1.7 homology model and demonstrate regions of high and low conservation in and around the central cavity.