Abstract
A clinical trial of radiotherapy with modified simultaneous integrated boost (SIB) technique against huge tumors was conducted. A 58-year-old male patient who had a huge pelvic tumor diagnosed as a rectal adenocarcinoma due to familial adenomatous polyposis was enrolled in this trial. The total dose of 77 Gy (equivalent dose in 2 Gy/fraction) and 64.5 Gy was delivered to the center of the tumor and the surrounding area respectively, and approximately 20% dose escalation was achieved with the modified SIB technique. The tumor with an initial maximum size of 15 cm disappeared 120 d after the start of the radiotherapy. Performance status of the patient improved from 4 to 0. Radiotherapy with modified SIB may be effective for patients with a huge tumor in terms of tumor shrinkage/disappearance, improvement of QOL, and prolongation of survival.
Keywords: Clinical trial, Image-guided radiotherapy, Rectal neoplasms, Quality of life, Neoplasm recurrence
Core tip: This paper introduces a new technique of radiation therapy for huge tumors. In the past, patients with radioresistant huge adenocarcinoma could undergo only palliative treatment because huge tumors could not be controlled with a dose less than tolerant dose of healthy tissue. However, this technique enabled to deliver higher-dose to the center of huge tumor without exceeding tolerant dose to the healthy tissue. Of course it is difficult to cure these patients, this technique showed a possibility to control huge tumors. From the patients enrolled in this clinical trial, we introduce a patient with tumor arising from digestive system.
INTRODUCTION
The incidence of colorectal cancer accounts for 10% of all new cancers, and the mortality caused by colorectal cancer accounts for 8% of all cancers[1]. Adenocarcinoma accounts for more than 95% of rectal cancers, with carcinoids, malignant lymphomas, squamous cell carcinomas, and others comprising the remaining 5%[2]. Familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, and hamartomatous polyposis syndromes are known causes of hereditary colorectal cancer, and a mutation of APC tumor suppressor gene is known cause of carcinogenesis[3]. Besides APC, a mutation of the K-ras gene or the p53 gene have a great influence on the development of colorectal cancer; moreover, a mutation or loss of the DCC gene may also affect the malignant progression of colorectal cancer[4-6]. On the other hand, alcohol drinking, smoking, lack of exercise, and obesity are known as acquired risk factors of colorectal cancer[7-11].
The standard treatment of a resectable rectal cancer is surgical resection: low anterior resection, Hartmann’s operation, or abdominoperineal resection are usually performed, and sometimes total mesorectal excision or autonomic nerves preservation are added for local control or function sparing[12,13]. In addition, adjuvant or neoadjuvant chemoradiotherapy is added for advanced rectal cancer to the recent standard treatment protocols[14]. Recent studies have reported that the 5-year local recurrence rate has decreased by 5%-15%, but locoregional failure is still the most frequent failure pattern[15]. Only palliative treatment usually is considered for unresectable or postoperative recurrent rectal cancer, and a standard definitive treatment for unresectable tumors has not yet been established[16].
The purpose of this study was to verify the efficacy of a new radiotherapy technique for huge refractory tumors.
CASE REPORT
Inclusion criteria of the patients
Patients assumed to have cancer originating from the digestive system were selected from among the patients who were enrolled in the clinical trial of the modified simultaneous integrated boost (SIB) radiotherapy for huge tumors. The main points of inclusion criteria of the clinical trial were as follows: (1) a histologically proven solid tumor; (2) a locally advanced or unresectable huge tumor; (3) the absence of a history of radiotherapy for the lesion to be treated; and (4) patients with informed consent.
Because of the design of the clinical trial, a limit was not set for the following points: the presence or absence of a history of surgical treatment/chemotherapy, combinations of anticancer drugs during radiotherapy, and the presence or absence of a distant metastasis at the start of radiotherapy. Patients with performance status of 0-4 could be enrolled in the clinical trial.
The exclusion criteria were as follows: (1) a history of radiotherapy for the lesion in question; (2) the absence of histological proof; (3) impossibility to maintain the necessary position during irradiation; (4) the absence of written consent; and (5) impossibility of the proposed treatment for some other reason.
Radiotherapy
The macroscopic extent of a tumor was defined as the gross tumor volume (GTV), whereas the area around GTV and the regional lymph node area were defined as clinical target volume (CTV). The area including setup margins around CTV was defined as the planning target volume (PTV). CT images for the radiotherapy planning (RTP) were obtained using a 16-row multidetector CT. RTP was supposed to be planned according to CT-based three-dimensional (3D) conformal planning. Both 3D conformal radiotherapy and intensity-modulated radiotherapy were allowed to be applied for the treatment planning. Radiotherapy plans were generated using treatment planning software with tissue inhomogeneity correction (XiO; ELEKTA, Japan). Irradiation was performed one fraction per day and five fractions per week using 4-10 MV photons by means of a high-energy linear accelerator. If there was respiratory motion of the target, the respiratory gating irradiation protocol was used. Image-guided radiotherapy with cone-beam CT or electric portal image detector was used for 3D setup before the irradiation.
Modified SIB technique
The conventional concept of radiotherapy is to irradiate uniformly within PTV. The concept of the general SIB technique is that there is heterogeneity in the dose distribution in PTV[17-19]. The dose delivered to GTV is increased, and the dose to the prophylactic area is set to a (lower) prophylactic dose. Although the dose distribution in PTV is not uniform, generally, the dose distribution in GTV is uniform in the general SIB technique (Figure 1A). The concept of the modified SIB technique (compared with the general SIB technique) is as follows: (1) the main point of this study was to deliver a higher dose to GTV as much as possible; (2) the dose to the healthy tissue is strictly limited to the tolerant dose; (3) the heterogeneity in the dose distribution within GTV is allowed to comply with the previous rules; and (4) the dose of boost irradiation to the GTV is defined as more than 10% of the dose to the surrounding healthy tissue in the cumulative dose; however, the upper limit of the dose to GTV has not been set (Figure 1B).
Figure 1.
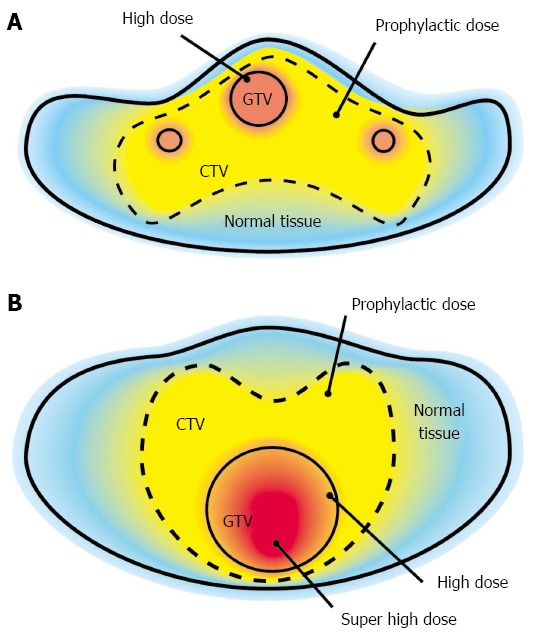
Simultaneous integrated boost irradiation. A: The concept behind standard simultaneous integrated boost (SIB) irradiation. A prophylactic dose is delivered to clinical target volume, and a curative (higher) dose is delivered to gross tumor volume (GTV). Dose distribution within GTV is usually homogeneous; B: The concept of the modified SIB irradiation technique. It is almost the same as the standard SIB technique, but the modified SIB technique can deliver a much higher dose to the part of GTV without excessive irradiation of the surrounding healthy tissue by allowing for heterogeneity of dose distribution within GTV.
An equivalent dose in 2 Gy per fraction (EQD2Gy) was calculated as follows: (total dose) × (dose/fraction +α/β)/(2+ α/β)[20]. The described dose shows a cumulative dose unless otherwise specified.
Evaluation procedures
The primary endpoint was a local response, and the secondary endpoints were defined as acute and late toxicity, QOL (quality of life), and survival period. The tumor response was evaluated by measuring the tumor size using imaging modalities such as CT, magnetic resonance imaging, positron emission tomography-CT, and ultrasonography). The overall survival period was measured from the first day of radiotherapy (day 1) to the final follow-up date. A recurrence-free survival period was measured from the first day of radiotherapy to the date of recurrence after radiotherapy. QOL was comprehensively evaluated based on subjective symptoms, objective symptoms, and ADL (activity of daily life). The toxicity was evaluated based on CTCAE version 4.0[21].
Patient
In all enrolled patients in this study, there was one patient with a tumor from gastrointestinal tract. A 58-year-old male patient with a huge pelvic tumor was enrolled in this study and treated with modified SIB radiotherapy. The patient had a history of a subtotal proctocolectomy and ileorectal anastomosis due to familial adenomatous polyposis when he was 27 years old. The patient developed a vesicorectal fistula at the age of 58 years, and then fistIulectomy, proctectomy, and ileostomy were performed. Histological examination of the resected vesicorectal fistula revealed adenocarcinoma and blood tests revealed high serum carcinoembryonic antigen (CEA) level; therefore, he was diagnosed with rectal cancer. Chemotherapy (FOLFOX4 × 4 courses, GEM+CDDP*2 courses) was performed after the diagnosis, but the tumor grew in size; therefore, the patient was prescribed radiotherapy.
Before the start of radiotherapy, there was a huge perineal tumor (9 cm × 11 cm × 15 cm) protruding to the surface (Figure 2 and Figure 3A). A loss of a part of the bladder wall because of tumor invasion could be seen in the sagittal image (Figure 2C and D), but there was no leakage of the urine because of obstruction by the huge tumor. The serum CEA level was 173.3 ng/mL (normal level < 5.0 ng/mL), and there were no other elevated tumor markers. The patient had strong cancer pain in the perineum despite large doses of oxycodone, and the patient’s performance status was determined as 4 because the patient could not walk or sit due to the cancer pain. There were objective symptoms of odor and blood oozing. Distant metastases had not been detected before radiotherapy.
Figure 2.
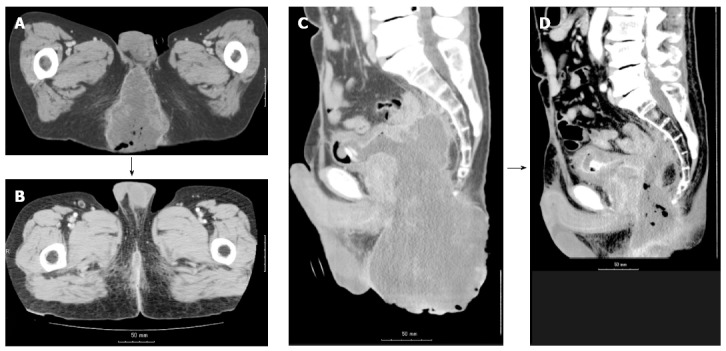
Computed tomography image. A: An axial computed tomography (CT) image before radiotherapy. A massive tumor is seen in the perineum, and a part of the tumor is protruding. Both lower extremities are elevated because the patient could not stretch both legs. B: An axial CT image on day 120 from the start of the radiotherapy. The macroscopic tumor disappeared, and the patient could stretch both lower extremities; C: A sagittal CT image before the radiotherapy; D: A part of the bladder wall has been lost to invasion of the massive solid tumor.
Figure 3.
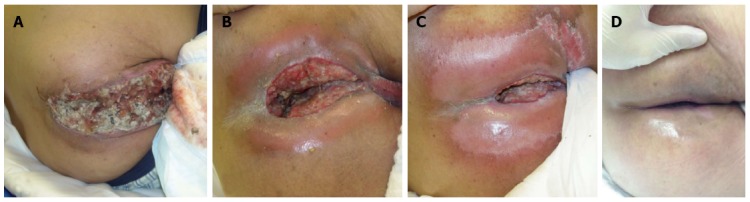
Macroscopic findings before radiotherapy. A: Day 0. The tumor is exposed from the perineum with oozing of blood, offensive odor and sever pain; B: Day 28 from the start of radiotherapy. The tumor was shrinking due to the modified SIB technique. Leakage of urine from a bladder fistula was observed; C: Day 56. Further tumor shrinkage is observed, but a slight residual tumor is present. The defect in the healthy tissue was shrinking; D: Day 180. The macroscopic tumor as well as exposure of the mucosa also disappeared. The patient could walk normally.
Treatment
RT plans for this patient are shown in Figure 4. Figure 4A shows a dose distribution of the initial RTP for this patient. This radiotherapy plan consisted of coplanar eight fields using the modified SIB technique. CTV (suspicious for microscopic invasion and regional lymph node area) was included in the volume of 1.8 Gy per fraction (yellow line), and 2.3 Gy per fraction was delivered to the central part of GTV (orange line). Nevertheless, not all of GTV was included in the high-dose area, and the dose to the border area between the tumor and healthy tissue was lowered to approximately 2 Gy; 36 Gy per 20 fractions and 46 Gy per 20 fractions were delivered to CTV and GTV respectively with this plan. After completing the initial course of radiotherapy, boost irradiation of GTV was performed according to the second radiotherapy plan using the shrinking field technique (not the modified SIB technique; Figure 4B). Furthermore, second boost irradiation of GTV was performed according to the third RT plan using the shrinking field technique (not the modified SIB technique; Figure 4C). Finally, a cumulative dose of 76.6 Gy per 37 fractions to the central part of GTV and a dose of 66.6 Gy per 37 fractions to CTV were delivered. EQD2Gy to the central part of GTV and that to CTV were calculated as approximately 77 Gy and 64.5 Gy respectively. The dose to the center of GTV was increased by 19.4% compared with the surrounding healthy tissue. With the management of cancer pain, the scheduled treatment was completed without any problems.
Figure 4.
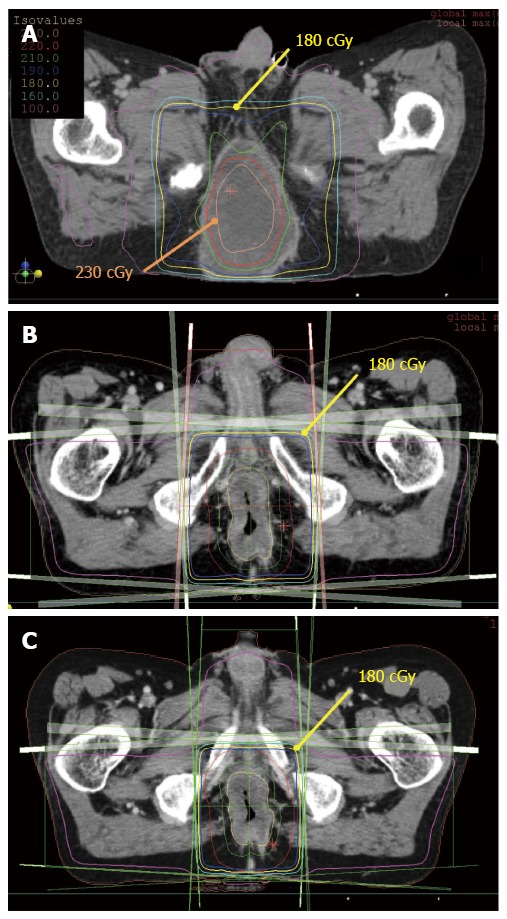
Radiotherapy planning. A: The initial radiotherapy planning (RTP). RTP consists of eight static fields using the modified simultaneous integrated boost (SIB) technique. The central part of gross tumor volume (GTV) is irradiated with 230 cGy per fraction. A total dose of 36 Gy per 20 fractions was delivered to the whole pelvis, and a total dose of 46 Gy per 20 fractions was delivered to a part of GTV; B: The second RTP for this patient (Usual boost irradiation. Not the modified SIB technique); C: The third RTP for this patient (not the modified SIB technique).
Tumor response
Figure 2 shows the tumor before radiotherapy (Figure 2A and B) and 70 d after the end of radiotherapy (Figure 2C and D). A macroscopic complete response (CR) was achieved. Although histopathological examination was not performed, there was no detectable tumor according to CT and the protruding macroscopic tumor disappeared. Serum CEA level increased to 202.7 ng/mL 30 d after the start of the radiotherapy, but it decreased to 12.4 ng/mL 70 d after the end of radiotherapy. Patient’s performance status and cancer pain improved as the treatment proceeded, and he became capable of easily maintaining the supine position during the irradiation 3-4 wk after the start of the radiotherapy. The blood oozing and offensive odor from the tumor disappeared along with disappearance of the tumor. The patient regained the ability to walk alone due to tumor disappearance and complete pain relief, and was discharged from the hospital in an ambulatory state after the completion of radiotherapy.
Outcomes
After the discharge from the hospital, the patient was followed up on an outpatient basis. Local recurrence was detected 270 d after the start of radiotherapy, and then chemotherapy and palliative treatment were administered. Eventually, the patient died of primary disease 1 year and 8 mo after the start of radiotherapy without treatment-related toxicity.
DISCUSSION
There are various clinical trials of multidisciplinary approaches to colorectal cancer. Intraoperative radiotherapy had been used for advanced rectal cancer to improve local controllability[22]. The chemotherapy drug 5-fluorouracil (5-FU) has been used for rectal cancer as the main chemotherapeutic agent to date[23]. The addition of oxaliplatin to the combination of 5-FU and leucovorin (FOLFOX) for the treatment of rectal cancer significantly improves disease-free survival and overall survival, according to a randomized controlled trial[24]. In addition, the combination containing irinotecan (FOLFIRI) was proved to be effective against advanced rectal cancer[25]. Although both pre- and postoperative RT are effective against advanced rectal cancer, preoperative RT is becoming a mainstream treatment according to the results of a randomized controlled trial comparing preoperative and postoperative RT[26]. Furthermore, the total toxicity of preoperative RT is smaller than that of postoperative RT[27,28]. However, it is thought that there is no difference among dose fractionation regimens (e.g., 25 Gy per 5 fractions per week, 50.4 Gy per 28 fractions per 5.5 wk) of preoperative RT.
To validate the existing evidence related to the treatment of advanced rectal cancer, a multidisciplinary approach is being established as preoperative chemotherapy consisting of 5-FU, LV, oxaliplatin, or irinotecan, and preoperative RT and surgery. In case of distant metastasis, molecularly targeted agents such as bevacizumab, cetuximab, and panitumumab can be used[29-31]. On the other hand, the standard treatment of chemotherapy-resistant unresectable rectal cancer has not been established yet. Generally, only palliative treatment is used for the patients with a far advanced cancer. This clinical trial has been organized to explore the possibility of radical treatment of such patients.
Tumor (local) response
As mentioned above, the goal of this study was to deliver a higher dose to a huge tumor than to the surrounding healthy tissue using the modified SIB technique and to evaluate the efficacy and toxicity. The calculated EQD2Gy to the center of GTV and to surrounding tissue was 77 Gy and 64.5 Gy respectively, and the dose to the center of GTV was 20% greater compared to the surrounding tissue.
According to the present results, macroscopic CR was obtained as a result of the proposed treatment. It is impossible to control a chemoresistant huge adenocarcinoma with a dose of approximately 65 Gy, but the local controllability increases with an increase in the dose by 20% (the total dose) to the center of GTV.
Clinical course/QOL
Blood oozing from the tumor resolved within a month from the start of the radiotherapy, and an almost complete relief of cancer pain in the perineum was also achieved within a month from the start of radiotherapy. Although radiotherapy would be stopped when a relief of symptoms were obtained in case of palliative treatment, in the present study, the radiotherapy was continued for the purpose of local control. The changes in the macroscopic findings of the huge tumor are shown in Figure 3. There was an exposed huge tumor with blood oozing and an offensive odor before the radiotherapy (Figure 3A). The tumor began to shrink, and there was a cavity due to tumor shrinkage on day 28 (Figure 3B) with almost no bleeding or odor.
Disappearance of the tumor and related symptoms
Thirty to 50 d after the start of the radiotherapy, transient urine leakage from the bladder fistula to the perineal cavity was observed. Although the loss of a part of the bladder wall due to tumor invasion was found before the radiotherapy (Figure 2B), urine leakage was not observed then because the tumor formed a part of the bladder wall, and the shrinkage of tumor size probably caused leakage (Figure 3B).
The clinical course such as this one is the inevitable problem when treating a T4 tumor. For example, the perforation of a healthy esophagus never occurs during the treatment of a T3 esophageal cancer with standard chemoradiotherapy, but it is sometimes observed due to tumor shrinkage when treating a T4 esophageal cancer[32-34]. We believe that the bladder fistula was due to a mechanism similar to that in the above examples; therefore, the fistula was considered as a cancer-related complication rather than treatment-related complication and radiotherapy was continued because the patient’s condition was stable without active bleeding or infection. After treatment completion, we observed that the cavity/fistula shrank, urine leakage disappeared, and perineal wound improved (Figure 3B-D). One of the differences between a loss of healthy tissue as a result of tumor invasion and a loss of healthy tissue caused by radiation-induced injury is the eventual healing of the wound. When a cancer disappears, the surrounding healthy tissue will begin to recover despite a loss of a part like in this case, and almost complete repair back to the normal status can be expected if the course is favorable[34]. According to these findings, we believe that a huge tumor should be treated aiming at local control even if it is a far-advanced T4 tumor.
Outcomes and toxicity
Local recurrence was observed 9 mo from the start of radiotherapy, and the patient died of the disease 1 year and 8 mo after the start of radiotherapy. Despite the transient macroscopic CR, long-term local control could not be achieved, unfortunately. On the other hand, given that the patient’s prognosis would have been 1-2 mo without radiotherapy with the modified SIB technique, it is possible that the modified SIB RT allowed the patient to survive for > 1 year with favorable ADL. Although the total dose to the part of GTV was increased by 20%, there was local recurrence without treatment-related toxicity. This result means that there is still room for dose escalation to the part of GTV. Furthermore, the transient tumor disappearance without distant metastases is suggestive of a possibility of a cure even for a far-advanced huge tumor like in this case.
In conclusion, radiotherapy using the modified SIB technique against a huge pelvic tumor was analyzed. It is impossible to deliver a much higher dose to the tumor with conventional radiotherapy, but radiotherapy using the modified SIB technique may successfully treat the center of GTV without significant toxicity. As a result, tumor disappearance, improvement of QOL/ADL, and prolongation of survival can be achieved. A computer assisted automatic segmentation of the target may be able to utilize for this treatment[35,36]. Accumulation of cases and validation of the technique are tasks for future research.
COMMENTS
Case characteristics
A huge adenocarcinoma arising in the pelvis was treated with radiotherapy using modified simultaneous integrated boost (SIB) technique.
Clinical diagnosis
A patient who had a history of subtotal colectomy due to familial adenomatous polyposis caused adenocarcinoma in the residual rectum, and was initially treated with chemotherapy regimens for rectal cancer.
Imaging diagnosis
A macroscopic tumor with erosion was exposed in the perineum, and computed tomography scan revealed a huge tumor in size of 9 cm × 11 cm × 15 cm with bladder invasion.
Pathological diagnosis
The tumor was diagnosed as adenocarcinoma by the needle biopsy.
Treatment
The tumor was treated with radiotherapy with a new technique that can irradiate much higher dose to the tumor without exceeding tolerance dose to the surrounding healthy tissue.
Related reports
Several authors have reported about “SIB” radiotherapy, but those are slightly different from “modified SIB” radiotherapy in the present study.
Term explanation
Modified simultaneous integrated boost (modified SIB) irradiation is a recent technique that can irradiate higher dose to a certain area in the irradiation field.
Experiences and lessons
It may be possible to obtain macroscopically complete response even against for huge adenocarcinomas with this irradiation technique.
Peer review
This is an interesting case, and to treat more patients is a problem in the future.
Footnotes
P- Reviewer: Casciaro S S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Kang H, O’Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183–189. doi: 10.1007/s00384-006-0145-2. [DOI] [PubMed] [Google Scholar]
- 3.Young J, Jenkins M, Parry S, Young B, Nancarrow D, English D, Giles G, Jass J. Serrated pathway colorectal cancer in the population: genetic consideration. Gut. 2007;56:1453–1459. doi: 10.1136/gut.2007.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Custodio A, Barriuso J, de Castro J, Martínez-Marín V, Moreno V, Rodríguez-Salas N, Feliu J. Molecular markers to predict outcome to antiangiogenic therapies in colorectal cancer: current evidence and future perspectives. Cancer Treat Rev. 2013;39:908–924. doi: 10.1016/j.ctrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: Anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154:37–49. doi: 10.7326/0003-4819-154-1-201101040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Mehlen P, Fearon ER. Role of the dependence receptor DCC in colorectal cancer pathogenesis. J Clin Oncol. 2004;22:3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Longnecker MP, Orza MJ, Adams ME, Vioque J, Chalmers TC. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control. 1990;1:59–68. doi: 10.1007/BF00053184. [DOI] [PubMed] [Google Scholar]
- 8.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 9.Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 10.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12:3186–3195. doi: 10.3748/wjg.v12.i20.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxter NN, Garcia-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014–1020. doi: 10.1200/JCO.2006.09.7840. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Rectal Cancer Version 3.2014. Available from: http://www.nccn:professionals/physician_gls/pdf/rectal.pdf.
- 15.Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, Lacasta A, Bujanda L. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674–1684. doi: 10.3748/wjg.v17.i13.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronnekleiv-Kelly SM, Kennedy GD. Management of stage IV rectal cancer: palliative options. World J Gastroenterol. 2011;17:835–847. doi: 10.3748/wjg.v17.i7.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peponi E, Glanzmann C, Kunz G, Renner C, Tomuschat K, Studer G. Simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT) in nasopharyngeal cancer. Strahlenther Onkol. 2010;186:135–142. doi: 10.1007/s00066-010-2048-y. [DOI] [PubMed] [Google Scholar]
- 18.Cho KH, Kim JY, Lee SH, Yoo H, Shin SH, Moon SH, Kim TH, Shin KH, Yoon M, Lee DH, et al. Simultaneous integrated boost intensity-modulated radiotherapy in patients with high-grade gliomas. Int J Radiat Oncol Biol Phys. 2010;78:390–397. doi: 10.1016/j.ijrobp.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Thilmann C, Zabel A, Grosser KH, Hoess A, Wannenmacher M, Debus J. Intensity-modulated radiotherapy with an integrated boost to the macroscopic tumor volume in the treatment of high-grade gliomas. Int J Cancer. 2001;96:341–349. doi: 10.1002/ijc.1042. [DOI] [PubMed] [Google Scholar]
- 20.Joiner MC. A simple alpha/beta-independent method to derive fully isoeffective schedules following changes in dose per fraction. Int J Radiat Oncol Biol Phys. 2004;58:871–875. doi: 10.1016/j.ijrobp.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. May 29, 2009 NIH publication # 09-7473. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 22.Hahnloser D, Haddock MG, Nelson H. Intraoperative radiotherapy in the multimodality approach to colorectal cancer. Surg Oncol Clin N Am. 2003;12:993–1013, ix. doi: 10.1016/s1055-3207(03)00091-7. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 24.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 25.Morse MA. Adjuvant therapy of colon cancer: current status and future developments. Clin Colon Rectal Surg. 2005;18:224–231. doi: 10.1055/s-2005-916283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927–1934. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, Malinowska M, Krynski J, Kosakowska E, Rutkowski A, Pietrzak L, Kepka L, Radziszewski J, et al. Neoadjuvant treatment for unresectable rectal cancer: an interim analysis of a multicentre randomized study. Radiother Oncol. 2013;107:171–177. doi: 10.1016/j.radonc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Engineer R, Mohandas KM, Shukla PJ, Shrikhande SV, Mahantshetty U, Chopra S, Goel M, Mehta S, Patil P, Ramadwar M, et al. Escalated radiation dose alone vs. concurrent chemoradiation for locally advanced and unresectable rectal cancers: results from phase II randomized study. Int J Colorectal Dis. 2013;28:959–966. doi: 10.1007/s00384-012-1630-4. [DOI] [PubMed] [Google Scholar]
- 29.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 30.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 31.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 32.Burt M, Diehl W, Martini N, Bains MS, Ginsberg RJ, McCormack PM, Rusch VW. Malignant esophagorespiratory fistula: management options and survival. Ann Thorac Surg. 1991;52:1222–128; discussion 1222-128;. doi: 10.1016/0003-4975(91)90005-b. [DOI] [PubMed] [Google Scholar]
- 33.Muto M, Ohtsu A, Miyamoto S, Muro K, Boku N, Ishikura S, Satake M, Ogino T, Tajiri H, Yoshida S. Concurrent chemoradiotherapy for esophageal carcinoma patients with malignant fistulae. Cancer. 1999;86:1406–1413. [PubMed] [Google Scholar]
- 34.Nomiya T, Teruyama K, Wada H, Nemoto K. Chemoradiotherapy for a patient with a giant esophageal fistula. World J Gastroenterol. 2007;13:2250–2254. doi: 10.3748/wjg.v13.i15.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Zarepisheh M, Uribe-Sanchez A, Moore K, Tian Z, Zhen X, Graves YJ, Gautier Q, Mell L, Zhou L, et al. Automatic treatment plan re-optimization for adaptive radiotherapy guided with the initial plan DVHs. Phys Med Biol. 2013;58:8725–8738. doi: 10.1088/0031-9155/58/24/8725. [DOI] [PubMed] [Google Scholar]
- 36.Conversano F, Franchini R, Demitri C, Massoptier L, Montagna F, Maffezzoli A, Malvasi A, Casciaro S. Hepatic vessel segmentation for 3D planning of liver surgery experimental evaluation of a new fully automatic algorithm. Acad Radiol. 2011;18:461–470. doi: 10.1016/j.acra.2010.11.015. [DOI] [PubMed] [Google Scholar]


