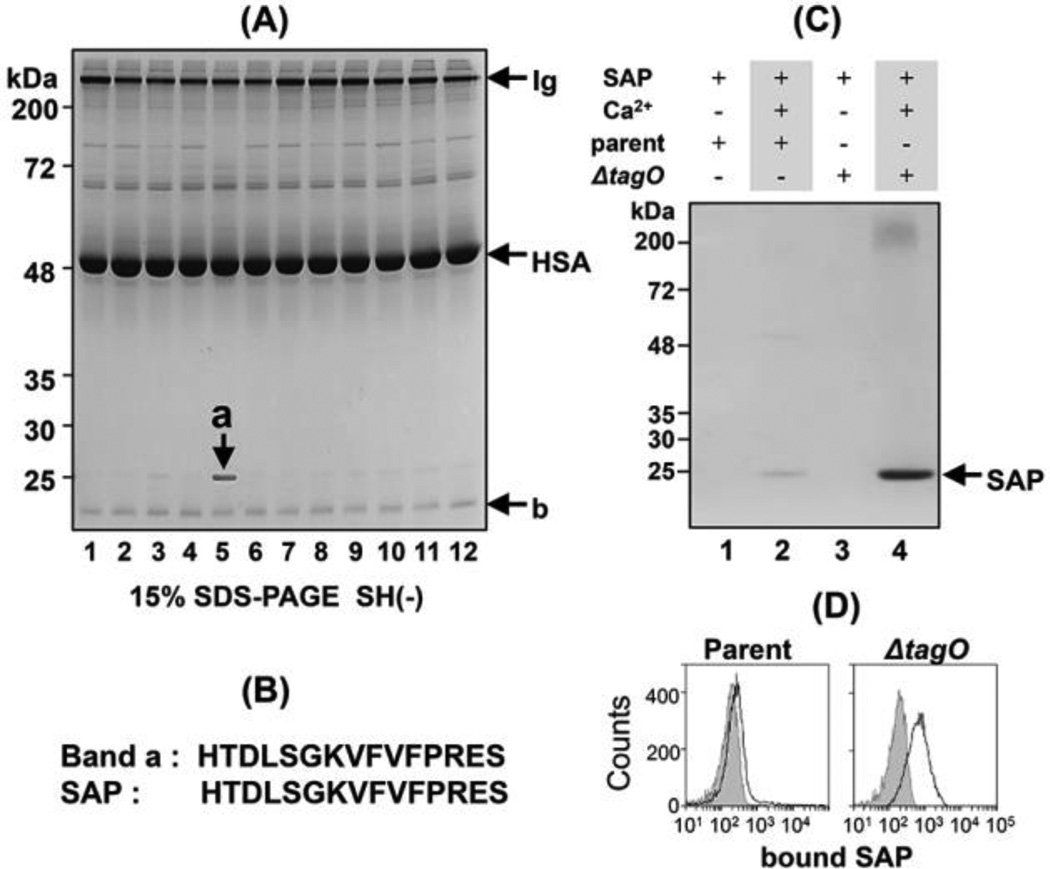
Figure 1. Biochemical characterization of a ΔtagO mutant-binding 25-kDa human serum protein.
(A) Screening of human serum for factors that bind S. aureus cell wall mutants. Twelve ethanol-fixed S. aureus cells (1.0 × 1010 cells) were incubated with 1 ml human serum. Cells were recovered by centrifugation and washed. Bound proteins were eluted with 0.1 M glycine (pH 2.5) and analyzed by SDS-PAGE on a 15% gel under non-reducing conditions. Protein bands were stained with Coomassie Brilliant Blue. The S. aureus mutants are described in Table 1. Lane 1, parental S. aureus; lane 2, Δspa; lane 3, ΔltaS, Δspa; lane 4, ΔltaS, Δspa/pSltaS; lane 5, ΔtagO, Δspa; lane 6, ΔtagO, Δspa/pStagO; lane 7, ΔoatA; lane 8, Δlgt; lane 9, ΔmprF; lane 10, ΔypfP; lane 11, ΔdltA; lane 12, ΔsrtA. The ΔtagO, Δspa double mutant sample (lane 5) was enriched for a 25-kDa serum protein, and when complemented with a tagO-expressing plasmid, this enrichment was not observed (lane 6). The arrow indicates SAP; HSA, human serum albumin; Ig, immunoglobulin. (B) Comparison of the N-terminal amino acid sequence of "band a” with that of human SAP. (C) Binding of SAP to the parental S. aureus RN4220 strain (parent) or the ΔtagO, Δspa double mutant (ΔtagO) in the presence or absence of calcium ion. (D) Binding of FITC-labeled SAP to the parental S. aureus RN4220 strain (parent) or the ΔtagO by flow cytometry as described in Materials and Methods. The curves with gray areas underneath represent cell-only controls.