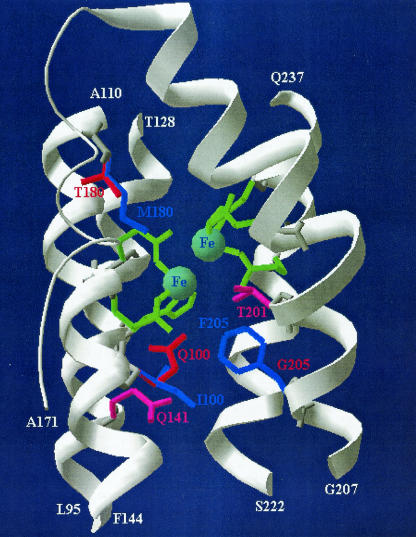
FIG. 4.
Active site of ToMO TouA alpha-subunit three-dimensional structure model constructed by using the crystal structure of sMMO from M. capsulatus (Bath) as the template. Residues in green (E110, E140, H143, E201, E235, and H238) are metal binding sites that form the diiron center (light blue). The I100Q, F205G, and M180T mutations are shown in red, and the wild-type I100, F205, and M180 residues are shown in blue. Saturation mutagenesis at Q141 and T201 resulted in no regiospecific mutants and is indicated by pink. Portions of the four-helix bundle of TouA (helix B, P87 to F117; helix C, P121 to K150; helix E, S185 to E214; and helix F, T219 to Q237) anchoring the diiron active site are shown in white; these regions end with L95 and A110 (helix B), T128 and F144 (helix C), A171 and G207 (loop plus helix E), and S222 and Q237 (helix F).