Abstract
The bicuspid aortic valve (BAV) is the most common form of inheritable cardiac defect. Although this abnormality may still achieve normal valvular function, it is often associated with secondary valvular and aortic complications such as calcific aortic valve disease and aortic dilation. The clinical significance and economic burden of BAV disease justify the need for improved clinical guidelines and more robust therapeutic modalities, which address the root-cause of those pathologies. Unfortunately, the etiology of BAV valvulopathy and aortopathy is still a debated issue. While the BAV anatomy and its secondary complications have been linked historically to a common genetic root, recent advances in medical imaging have demonstrated the existence of altered hemodynamics near BAV leaflets prone to calcification and BAV aortic regions vulnerable to dilation. The abnormal mechanical stresses imposed by the BAV on its leaflets and on the aortic wall could be transduced into cell-mediated processes, leading ultimately to valvular calcification and aortic medial degeneration. Despite increasing evidence for this hemodynamic etiology, the demonstration of the involvement of mechanical abnormalities in the pathogenesis of BAV disease requires the investigation of causality between the blood flow environment imposed on the leaflets and the aortic wall and the local biology, which has been lacking to date. This editorial discusses the different hypothetical etiologies of BAV disease with a particular focus on the most recent advances in cardiovascular imaging, flow characterization techniques and tissue culture methodologies that have provided new evidence in support of the hemodynamic theory.
Keywords: Aortopathy, Valvulopathy, Hemodynamics, Bicuspid aortic valve, Shear stress
Core tip: The bicuspid aortic valve (BAV) is associated with secondary aortopathy and valvulopathy. However, the root cause of those complications remains controversial. While the genetic etiology has been the most popular historically, advances in cardiovascular imaging, flow characterization and tissue culture methodologies have provided new evidence in support of a hemodynamic origin. The assessment of the respective role of genetic and hemodynamic cues in BAV pathogenesis is critical to the development of improved diagnosis tools and patient-specific modalities. This editorial discusses the different possible etiologies of BAV disease with a particular focus on the most recent evidence for the hemodynamic pathway.
INTRODUCTION
Despite a limited prevalence of 1%-2% in the general population[1-3], the bicuspid aortic valve (BAV) is the most common inheritable valvular defect. As compared to a normal tricuspid aortic valve (TAV), which consists of three leaflets, the BAV forms with only two as a result of cusp fusion during development. The BAV exists in different morphologic phenotypes[4-6]. The most prevalent type-I morphology features two cusps of unequal size and a fibrous raphe at the location of congenital fusion[7-9]. While 71% of type-I BAVs result from the fusion between the right- and left-coronary leaflets (LR subtype), 15% feature right- and non-coronary cusp fusion (RN subtype) and 3% present with non- and left-coronary cusp fusion (NL subtype)[7]. The BAV has emerged as the most common indication for surgical valvular replacement and is often complicated by secondary valvular and aortic wall abnormalities such as calcific aortic valve disease (CAVD) and aortic dilation, respectively[3,10-17]. The current management of BAV disease presents significant challenges related to the nontrivial early identification of BAV patients, the difficult detection of the onset of aortic and valvular complications and the time-sensitivity of surgical intervention[18]. The development of improved clinical guidelines and more robust therapeutic modalities addressing the root-cause of BAV disease requires the knowledge of the etiology of those pathologies, which is still under debate. This editorial discusses recent clinical and bioengineering developments in support of the hemodynamic theory of BAV disease, future research needs and their potential impact on clinical management.
BICUSPID AORTIC VALVE DISEASE
CAVD
Formerly considered a passive age-related disease promoted by cardiovascular risk factors and genetic predispositions, CAVD is now recognized as an active disease process involving inflammatory, extracellular matrix remodeling and osteogenic mediators as well as phenotypic changes in the valve interstitial cell population[19-22]. The later stage is characterized by formation of calcium nodules preferentially on the fibrosa (i.e., leaflet aortic surface)[23]. The stenosis caused by the stiffening of the leaflet tissue imposes a pressure overload on the left ventricle, which may result in hypertrophy and ultimately lead to heart failure[24]. While those disease features are common to both TAVs and BAVs, the valve anatomy is a strong predictor of the prevalence and progression of the disease. In the TAV population, CAVD affects about 25% of individuals above 65 years of age and the progression of the disease is relatively slow (20-30 years before severe valvular stenosis can be detected)[20,25-27]. In contrast, it is estimated that 40%-53% of BAV patients develop some form of CAVD and that it may take as little as 10-12 years for the disease to result in severe valvular stenosis[16,17].
Aortic dilation
Aortic dilation is another common complication in BAV patients[10,14,28-31]. This condition, which characterizes the gradual thinning of the aortic wall and the enlargement of the aortic lumen above 4.0 cm in diameter[32], is a precursor event to dissection and ultimate rupture. The dilation of the thoracic ascending aorta downstream of a BAV is marked by structural wall abnormalities including aortic medial degradation, smooth muscle cell apoptosis and depletion, elastic fiber degeneration and abnormal extracellular remodeling[31], which localize primarily to the convexity of the aortic wall[33,34]. The particular BAV morphotype has also been shown to affect the pattern of dilation. The LR type-I BAV subtype is associated with a larger annulus and sinus than the RN phenotype[35]. While aortic dilation generally spares TAV patients and only occurs in 12%-22% of those with hypertension[36,37], it affects between 35%-68% of BAV patients[16,17,31,38-41]. In addition, while TAV ascending aortas typically experience a dilation rate of 0.07-0.2 mm/year, BAV patients experience much more rapid progression of dilation at rates of 0.2-1.9 mm/year[42,43]. As a result, acute aortic dissection occurs 5 to 10 times more frequently and at an earlier age in BAV patients than in TAV patients[3,10,44-46].
HYPOTHETICAL ETIOLOGIES AND KNOWLEDGE GAP
While the genetic root of the BAV malformation has been clearly demonstrated, the etiology of BAV disease is still a matter of debate[47-49]. The most accepted genetic theory hypothesizes that the abnormal valve structure, the vulnerability of BAV leaflets to calcification and the BAV aorta to dilation originate from a common congenital defect. Supporting evidence for this etiology stems from the apparent heritability of the BAV defect[50,51] and the association between certain gene mutations (e.g., GATA5, NOTCH1, ACTA2), leaflet calcification and aortic dilation[52-55].
The less popular hemodynamic theory considers the mechanical stresses produced by the abnormal valve anatomy as the driving factor of secondary valvulopathy and aortopathy. Evidence for this mechano-etiology is supported by the apparent correlation between the eccentric BAV orifice jet[56-59], the presentation of aortic dilation in wall regions subjected to wall shear stress overload[33,34] and the preferential formation of calcific nodules on the fused BAV leaflet, which experiences a higher degree of wall shear stress abnormality relative to the non-fused leaflet[3-5,60]. Despite those observations, the validation of the hemodynamic etiology of BAV disease requires demonstration of causality, which to date has been lacking.
EVIDENCE FOR A HEMODYNAMIC PATHWAY
The mechanistic elucidation of the role played by hemodynamics in BAV disease requires the implementation of integrative approaches that not only describe the genetics of valvular morphogenesis and the impact of the BAV anatomy on valvular function but also investigate the adaptive and pathological responses of valvular and aortic cells to the native BAV flow environment. The emergence of state-of-the-art flow measurement and modeling tools combined with advanced tissue conditioning systems have provided a unique opportunity to examine ex vivo the role played by BAV hemodynamics, in the absence of underlying genetic defects and concurrent risk factors.
The implementation of advanced clinical and engineering tools toward the quantification of BAV flow has provided new insights into the hemodynamic complexity of this valvular defect and detailed maps of the wall shear stress abnormalities experienced by BAV leaflets and the BAV ascending aorta. In vitro particle-image velocimetry measurements in porcine valve models demonstrated the existence of an elliptical valve orifice, an eccentric jet skewed toward the non-coronary leaflet and an intrinsic degree of stenosis in type-I BAVs[61-63]. Pulsatile fluid-structure interaction simulations in valve and ascending aorta models[64,65], phase-contrast magnetic resonance imaging[59,66] and cardiovascular magnetic resonance[67,68] isolated dramatic differences in the frequency and magnitude of the wall shear stress generated on the leaflets and ascending aorta downstream of a TAV and a type-I BAV. Those modalities identified the fused BAV leaflet and the convex region of the BAV ascending aorta as those experiencing the highest degree of wall shear stress abnormality as compared to their TAV counterparts.
The concurrent development of sophisticated tissue culture systems capable of subjecting native BAV leaflets[69] or aortas[70] to their native local hemodynamic stress environment has enabled the rigorous investigation of the isolated effects of BAV flow on valvular calcification and aortic dilation. Those mechanobiological studies demonstrated for the first time: (1) the ability of the wall shear stress overload present on the convexity of LR type-I BAV ascending aortas to promote locally aortic medial degradation via matrix metalloproteinase-dependent pathways[65,71]; and (2) the particular susceptibility of the wall shear stress generated on the fused LR type-I BAV leaflet to trigger early calcification events such as endothelial activation, paracrine signaling, extracellular matrix degradation and bone matrix synthesis[72,73].
Collectively, those observations support a hemodynamic etiology by which the abnormal mechanical stresses experienced by BAV leaflets and BAV ascending aortas could trigger molecular pathways leading to the progressive calcification of the leaflets and the weakening of the aortic wall. Specifically, the hemodynamic theory postulates that the abnormal BAV anatomy subjects the valve leaflets and the ascending aortic wall to local stress overloads, which can be sensed by specific receptors in the tissue endothelium and then transduced into different pathological responses that would lead ultimately to the formation of calcific nodules on the leaflets or the progressive degeneration of the aortic media (Figure 1). The amplification of the degree of hemodynamic abnormality caused by the gradual stiffening of the leaflets and dilation of the proximal aorta may result in turn in the amplification of the pathological cascade and the acceleration of the disease process.
Figure 1.
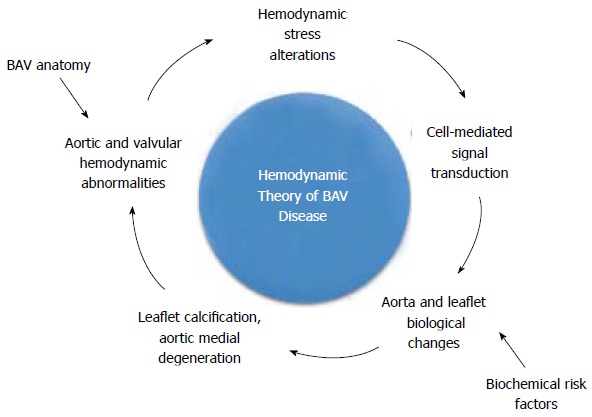
Hemodynamic theory of bicuspid aortic valve disease. The hemodynamic theory of bicuspid aortic valve (BAV) disease can be illustrated as an irreversible feedback loop in which the BAV anatomy would subject the valve leaflets and the ascending aortic wall to local stress overloads. Those stress abnormalities could be sensed by specific endothelial receptors and then transduced into different biological responses that would lead ultimately to the formation of calcific lesions on the leaflets or to the progressive degeneration of the aortic media.
POTENTIAL CLINICAL IMPACTS
Whether and how hemodynamic cues contribute to BAV disease are important research questions that need to be addressed thoroughly in order to design improved diagnostic tools and more effective practice guidelines. As recognized by the Heart, Lung and Blood Institute (National Institutes of Health), the current state of the science on BAV disease does not permit to support particular pharmacological targets[74]. The effectiveness of the pharmacological approach depends on the ability to identify target molecules involved in the early stage of BAV disease before calcification and aortic medial degradation attain a point of no return. Therefore, the elucidation of the role played by hemodynamics in BAV valvulopathy and aortopathy may become instrumental to the future development of targeted cellular therapies as it has the potential to identify candidate mechano-sensitive molecules that may play a significant role in the initiation of BAV disease.
The current practice guidelines for the management of BAV complications[75] have been developed based on the prevailing theory of their etiology, which, historically, has been the genetic theory[47,48,53]. The limited understanding of the pathogenesis of BAV calcification and aortic dilation combined with the lack of pharmacological targets has driven the development of aggressive surgical procedures aimed at recovering valvular function[76,77] and eliminating the weakened ascending aortic wall[78,79]. While such strategies may be appropriate to address a congenital disease, it may not be as effective should the formation of valvular calcific lesions or the structural degeneration of the aortic wall be the result of adaptive mechanisms to the abnormal BAV flow. In this context, the involvement of flow-mediated signaling pathways in BAV aortopathy and valvulopathy should not be ignored as they may guide the development of new therapeutic modalities aimed at normalizing BAV flow or inhibiting pharmacologically the valvular and aortic pathological cascades at an early age.
Lastly, current diagnosis techniques for BAV calcification and aortic dilation rely on criteria that can only be assessed at an advanced stage of the disease. The exploration of improved techniques enabling early detection in young patients requires the fundamental knowledge of the disease mechanisms. More importantly, the modeling of those mechanisms in individual patients could provide predictive capabilities that will transform clinical decision-making and personalized care. Therefore, the demonstration of cause-and-effects relationships between BAV hemodynamics, valvular calcification and aortic wall degeneration may lay the foundations for computer-based predictive models of BAV disease by integrating the mathematical formulation of flow-sensitive valvular and vascular biological pathways in patient-specific flow models. Such models will help predict disease onset and progression and guide the choice of the optimal treatment strategy.
FUTURE DIRECTIONS
The novel therapeutic perspectives discussed above suggest several recommendations for future research. The increasing need for understanding the pathogenesis of BAV disease motivates the investigation of potential links between BAV morphotype, genotype and hemodynamics, and the detailed description of the key features that stimulate BAV calcification and aortic dilation.
In addition, new emphasis should be put toward the elucidation of the basic biology of BAV disease from early events to long-term mechanisms. Those efforts would involve the characterization of the signaling pathways of BAV calcification and aortic dilation, as well as the multi-scale description of the synergistic effects between valvular calcification, aortic medial degeneration, micro-scale mechanotransduction and macro-scale hemodynamics.
The current evidence in support of the hemodynamic theory has been provided by short-term ex vivo studies and small-scale clinical investigations. The development of improved laboratory methodologies enabling prolonged tissue exposure to BAV hemodynamics while maintaining tissue sterility and integrity may shed some light on how the acute adaptive mechanisms reported thus far evolve in the long-term. Clinical investigations on large BAV patient populations will also permit to determine the validity of the hemodynamic and genetic theories in a more statistically significant way.
CONCLUSION
In summary, evidence for the causative effects of BAV hemodynamics on secondary valvulopathy and aortopathy is emerging. While those complications may still be promoted by some genetic predispositions, it is likely that their pathogenesis is also driven by synergies between the local mechanical stress abnormalities and the local biology of the leaflets and ascending aortic wall. Long-term ex vivo studies and large-scale clinical investigations are needed to assess the respective contribution of the genetic and hemodynamic pathways and to determine the full spectrum of mechano-sensitive processes triggered by BAV hemodynamics. The new knowledge gained from those efforts may enable the development of improved diagnosis tools and therapeutic modalities capable of addressing the root cause of BAV disease.
Footnotes
Supported by National Science Foundation faculty early CAREER grant, No. CMMI-1148558; National Science Foundation Graduate Research Fellowship, No. 1000082474; and American Heart Association Predoctoral Fellowship, No. 14PRE18940010
P- Reviewer: Forte A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 5.Sabet HY, Edwards WD, Tazelaar HD, Daly RC. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74:14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes SM, Sanders SP, Khairy P, Jenkins KJ, Gauvreau K, Lang P, Simonds H, Colan SD. Morphology of bicuspid aortic valve in children and adolescents. J Am Coll Cardiol. 2004;44:1648–1651. doi: 10.1016/j.jacc.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 7.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Braverman AC, Güven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.De Mozzi P, Longo UG, Galanti G, Maffulli N. Bicuspid aortic valve: a literature review and its impact on sport activity. Br Med Bull. 2008;85:63–85. doi: 10.1093/bmb/ldn002. [DOI] [PubMed] [Google Scholar]
- 10.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, Eagle KA, Mehta RH, Nienaber CA, Pape LA. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–669. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 11.Epperlein S, Mohr-Kahaly S, Erbel R, Kearney P, Meyer J. Aorta and aortic valve morphologies predisposing to aortic dissection. An in vivo assessment with transoesophageal echocardiography. Eur Heart J. 1994;15:1520–1527. doi: 10.1093/oxfordjournals.eurheartj.a060424. [DOI] [PubMed] [Google Scholar]
- 12.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–855. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 13.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102:III35–III39. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 14.Nkomo VT, Enriquez-Sarano M, Ammash NM, Melton LJ, Bailey KR, Desjardins V, Horn RA, Tajik AJ. Bicuspid aortic valve associated with aortic dilatation: a community-based study. Arterioscler Thromb Vasc Biol. 2003;23:351–356. doi: 10.1161/01.atv.0000055441.28842.0a. [DOI] [PubMed] [Google Scholar]
- 15.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 16.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- 17.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 18.Vallely MP, Semsarian C, Bannon PG. Management of the ascending aorta in patients with bicuspid aortic valve disease. Heart Lung Circ. 2008;17:357–363. doi: 10.1016/j.hlc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Otto CM. Valvular Heart Disease. 2nd ed. Philadelphia, PA: Saunders; 2004. [Google Scholar]
- 20.O’Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 21.Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 23.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 24.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors--a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 25.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 26.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 27.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 28.Khoo C, Cheung C, Jue J. Patterns of aortic dilatation in bicuspid aortic valve-associated aortopathy. J Am Soc Echocardiogr. 2013;26:600–605. doi: 10.1016/j.echo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 29.McKusick VA. Association of congenital bicuspid aortic valve and erdheim’s cystic medial necrosis. Lancet. 1972;1:1026–1027. doi: 10.1016/s0140-6736(72)91211-1. [DOI] [PubMed] [Google Scholar]
- 30.Bonderman D, Gharehbaghi-Schnell E, Wollenek G, Maurer G, Baumgartner H, Lang IM. Mechanisms underlying aortic dilatation in congenital aortic valve malformation. Circulation. 1999;99:2138–2143. doi: 10.1161/01.cir.99.16.2138. [DOI] [PubMed] [Google Scholar]
- 31.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 32.Lu MT, Thadani SR, Hope MD. Quantitative assessment of asymmetric aortic dilation with valve-related aortic disease. Acad Radiol. 2013;20:10–15. doi: 10.1016/j.acra.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, Amarelli C, Scardone M, Di Meglio F, Guerra G, et al. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130:504–511. doi: 10.1016/j.jtcvs.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Della Corte A, Quarto C, Bancone C, Castaldo C, Di Meglio F, Nurzynska D, De Santo LS, De Feo M, Scardone M, Montagnani S, et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg. 2008;135:8–18, 18.e1-2. doi: 10.1016/j.jtcvs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, Otto CM. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–1638. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 36.Cuspidi C, Meani S, Fusi V, Valerio C, Sala C, Zanchetti A. Prevalence and correlates of aortic root dilatation in patients with essential hypertension: relationship with cardiac and extracardiac target organ damage. J Hypertens. 2006;24:573–580. doi: 10.1097/01.hjh.0000209992.48928.1f. [DOI] [PubMed] [Google Scholar]
- 37.Milan A, Avenatti E, Tosello F, Iannaccone A, Leone D, Magnino C, Veglio F. Aortic root dilatation in essential hypertension: prevalence according to new reference values. J Hypertens. 2013;31:1189–1195. doi: 10.1097/HJH.0b013e32835f8fda. [DOI] [PubMed] [Google Scholar]
- 38.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 39.Hahn RT, Roman MJ, Mogtader AH, Devereux RB. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. 1992;19:283–288. doi: 10.1016/0735-1097(92)90479-7. [DOI] [PubMed] [Google Scholar]
- 40.Nistri S, Sorbo MD, Marin M, Palisi M, Scognamiglio R, Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beroukhim RS, Kruzick TL, Taylor AL, Gao D, Yetman AT. Progression of aortic dilation in children with a functionally normal bicuspid aortic valve. Am J Cardiol. 2006;98:828–830. doi: 10.1016/j.amjcard.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 42.La Canna G, Ficarra E, Tsagalau E, Nardi M, Morandini A, Chieffo A, Maisano F, Alfieri O. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol. 2006;98:249–253. doi: 10.1016/j.amjcard.2006.01.096. [DOI] [PubMed] [Google Scholar]
- 43.Patel HJ, Deeb GM. Ascending and arch aorta: pathology, natural history, and treatment. Circulation. 2008;118:188–195. doi: 10.1161/CIRCULATIONAHA.107.690933. [DOI] [PubMed] [Google Scholar]
- 44.Edwards WD, Leaf DS, Edwards JE. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation. 1978;57:1022–1025. doi: 10.1161/01.cir.57.5.1022. [DOI] [PubMed] [Google Scholar]
- 45.Roberts CS, Roberts WC. Dissection of the aorta associated with congenital malformation of the aortic valve. J Am Coll Cardiol. 1991;17:712–716. doi: 10.1016/s0735-1097(10)80188-3. [DOI] [PubMed] [Google Scholar]
- 46.Losenno KL, Goodman RL, Chu MW. Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol Res Pract. 2012;2012:145202. doi: 10.1155/2012/145202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girdauskas E, Borger MA, Secknus MA, Girdauskas G, Kuntze T. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39:809–814. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Barker AJ, Markl M. The role of hemodynamics in bicuspid aortic valve disease. Eur J Cardiothorac Surg. 2011;39:805–806. doi: 10.1016/j.ejcts.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bossé Y, Limongelli G, Bossone E, Benson DW, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon) Circulation. 2014;129:2691–2704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 51.Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J Am Coll Cardiol. 1997;30:1809–1812. doi: 10.1016/s0735-1097(97)00372-0. [DOI] [PubMed] [Google Scholar]
- 52.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009;53:2288–2295. doi: 10.1016/j.jacc.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 54.Padang R, Bannon PG, Jeremy R, Richmond DR, Semsarian C, Vallely M, Wilson M, Yan TD. The genetic and molecular basis of bicuspid aortic valve associated thoracic aortopathy: a link to phenotype heterogeneity. Ann Cardiothorac Surg. 2013;2:83–91. doi: 10.3978/j.issn.2225-319X.2012.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padang R, Bagnall RD, Richmond DR, Bannon PG, Semsarian C. Rare non-synonymous variations in the transcriptional activation domains of GATA5 in bicuspid aortic valve disease. J Mol Cell Cardiol. 2012;53:277–281. doi: 10.1016/j.yjmcc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Girdauskas E, Disha K, Borger MA, Kuntze T. Relation of bicuspid aortic valve morphology to the dilatation pattern of the proximal aorta: focus on the transvalvular flow. Cardiol Res Pract. 2012;2012:478259. doi: 10.1155/2012/478259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathan DP, Xu C, Plappert T, Desjardins B, Gorman JH, Bavaria JE, Gorman RC, Chandran KB, Jackson BM. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg. 2011;92:1384–1389. doi: 10.1016/j.athoracsur.2011.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bissell MM, Hess AT, Biasiolli L, Glaze SJ, Loudon M, Pitcher A, Davis A, Prendergast B, Markl M, Barker AJ, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6:499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahadevia R, Barker AJ, Schnell S, Entezari P, Kansal P, Fedak PW, Malaisrie SC, McCarthy P, Collins J, Carr J, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129:673–682. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewin MB, Otto CM. The bicuspid aortic valve: adverse outcomes from infancy to old age. Circulation. 2005;111:832–834. doi: 10.1161/01.CIR.0000157137.59691.0B. [DOI] [PubMed] [Google Scholar]
- 61.Saikrishnan N, Yap CH, Milligan NC, Vasilyev NV, Yoganathan AP. In vitro characterization of bicuspid aortic valve hemodynamics using particle image velocimetry. Ann Biomed Eng. 2012;40:1760–1775. doi: 10.1007/s10439-012-0527-2. [DOI] [PubMed] [Google Scholar]
- 62.Seaman C, Akingba AG, Sucosky P. Steady flow hemodynamic and energy loss measurements in normal and simulated calcified tricuspid and bicuspid aortic valves. J Biomech Eng. 2014:136. doi: 10.1115/1.4026575. [DOI] [PubMed] [Google Scholar]
- 63.Seaman C, Sucosky P. Anatomic versus effective orifice area in a bicuspid aortic valve. Echocardiography. 2014;31:1028. doi: 10.1111/echo.12720. [DOI] [PubMed] [Google Scholar]
- 64.Chandra S, Rajamannan NM, Sucosky P. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech Model Mechanobiol. 2012;11:1085–1096. doi: 10.1007/s10237-012-0375-x. [DOI] [PubMed] [Google Scholar]
- 65.Atkins SK, Cao K, Rajamannan NM, Sucosky P. Bicuspid aortic valve hemodynamics induces abnormal medial remodeling in the convexity of porcine ascending aortas. Biomech Model Mechanobiol. 2014;13:1209–1225. doi: 10.1007/s10237-014-0567-7. [DOI] [PubMed] [Google Scholar]
- 66.Hope MD, Sigovan M, Wrenn SJ, Saloner D, Dyverfeldt P. MRI hemodynamic markers of progressive bicuspid aortic valve-related aortic disease. J Magn Reson Imaging. 2014;40:140–145. doi: 10.1002/jmri.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hope MD, Hope TA, Crook SE, Ordovas KG, Urbania TH, Alley MT, Higgins CB. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging. 2011;4:781–787. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Barker AJ, Markl M, Bürk J, Lorenz R, Bock J, Bauer S, Schulz-Menger J, von Knobelsdorff-Brenkenhoff F. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5:457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 69.Sun L, Rajamannan NM, Sucosky P. Design and validation of a novel bioreactor to subject aortic valve leaflets to side-specific shear stress. Ann Biomed Eng. 2011;39:2174–2185. doi: 10.1007/s10439-011-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sucosky P, Padala M, Elhammali A, Balachandran K, Jo H, Yoganathan AP. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng. 2008;130:035001. doi: 10.1115/1.2907753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sucosky P. Hemodynamic Mechanisms of Bicuspid Aortic Valve Calcification and Aortopathy. In: Rajamannan N, editor. Molecular Biology of Valvular Heart Disease. London: Springer; 2014. pp. 81–94. [Google Scholar]
- 72.Sun L, Chandra S, Sucosky P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS One. 2012;7:e48843. doi: 10.1371/journal.pone.0048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sucosky P, Rajamannan NM. Bicuspid Aortic Valve Disease: From Bench to Bedside. In: Rajamannan N, editor. Cardiac Valvular Medicine. London: Springer; 2013. pp. 17–21. [Google Scholar]
- 74.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonow RO, Carabello BA, Kanu C, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 76.Sheikh AM, Livesey SA. Surgical management of valve disease in the early 21st century. Clin Med. 2010;10:177–181. doi: 10.7861/clinmedicine.10-2-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verma S, Yanagawa B, Kalra S, Ruel M, Peterson MD, Yamashita MH, Fagan A, Currie ME, White CW, Wai Sang SL, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg. 2013;146:1033–1040.e4. doi: 10.1016/j.jtcvs.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 79.Conaglen P, Luthra S, Skillington P. Comparison of reduction ascending aortoplasty and ascending aortic replacement for bicuspid valve related aortopathy in young adult patients undergoing aortic valve replacement--long-term follow-up. Heart Lung Circ. 2009;18:337–342. doi: 10.1016/j.hlc.2009.03.049. [DOI] [PubMed] [Google Scholar]


