Abstract
Background: The methylene tetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with hypertension in certain populations. This study investigated the relationship between the MTHFR polymorphism and hypertension and correlated blood lipid indexes, including homocysteine (HCY), lipoprotein (a) [Lp (a)], high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A I (Apo AI), Apo B, glucose (GLU), total cholesterol (TC), and triglyceride (TG), in a Chinese population. Materials and Methods: A total of 174 patients with hypertension and 634 healthy control individuals from Jiangxi Province were recruited between June 2012 and September 2012 for genotyping of the MTHFR C677T polymorphism using polymerase chain reaction-restriction fragment length polymorphism. Biochemical parameters were also assessed in these subjects and statistically compared to the MTHFR C677T polymorphism and the risk for hypertension. Results: HCY and Lp (a) levels were significantly higher in subjects with a MTHFR 677TT genotype than in those with a CC/CT genotype, independent of hypertension. The frequency of the TT genotype and the T allele in hypertension patients was significantly higher than in the healthy controls. Furthermore, in the male hypertension patient group, the average levels of HCY, HDL, Apo AI, and TC were significantly different from those in female hypertension patients (pHCY=0.001, pHDL=0.004, pApo AI<0.001, pTC=0.012). In the male control group, the average levels of HCY, HDL, Apo AI, GLU, and TC were significantly different from those of female controls (pHCY<0.001, pHDL<0.001, pApo AI<0.001, pGLU=0.001, and pTC=0.004). Conclusion: Our data demonstrate that the MTHFR C677T polymorphism is positively correlated with an increased risk of hypertension through an increase in HCY levels. The blood lipid correlative index was different between male and female hypertension patients and controls.
Introduction
Cardiovascular disease accounted for 30% of the 17 million global deaths in 2008 (Ala et al., 2011). Hypertension and dyslipidemia are two major risk factors for cardiovascular disease (Ala et al., 2011). Cardiovascular disease is frequently treated with medication and surgery. However, understanding the underlying mechanisms of cardiovascular disease may help to reduce disease incidence and to help prevention in worldwide populations. To this end, research regarding genetic and environmental interactions has indicated that individuals with specific gene polymorphisms have an increased risk for developing cardiovascular disease. For example, some studies indicate that the methylene tetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with increased risk of cardiovascular disease and hypertension (Gupta et al., 2012; Bayramoglu et al., 2013; Kang et al., 2013; Wang et al., 2013; Xu et al., 2013; Zhao and Jiang, 2013), although other reports have failed to confirm this association (Davis et al., 2013). The MTHFR protein plays an important role in maintaining levels of folate and methionine, as well as controlling the blood concentration of homocysteine (HCY) in the human body (Wilson et al., 2010). The MTHFR C677T polymorphism results in mutated CT or TT genotypes, leading to a decreased enzyme activity when compared to the wild-type (CC) enzyme (Taioli et al., 2009).
Alteration in the blood lipid chemical index, including HCY, lipoprotein (a) [Lp (a)], high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A I (Apo AI), Apo B, glucose (GLU), total cholesterol (TC), and triglyceride (TG) levels, can increase the risk of developing hypertension and cardiovascular disease. HCY is an important intermediate in human sulfur-containing amino acid metabolism and is the product of S-adenosine homocysteine hydrolysis in the methionine cycle. HCY contributes to the remethylation and trans-sulfuration pathways. Remethylation is the primary pathway of HCY metabolism, in which HCY is catalyzed by methionine synthase. The pathway requires vitamin and 5-methyl-tetrahydrofolate to synthesize tetrahydrofolate and methionine. Alteration of this pathway is associated with cardiovascular, neurodegenerative, and kidney disorders (Welch and Loscalzo, 1998; Hankey and Eikelboom, 1999; Smulders and Blom, 2011; Malinowska et al., 2012) and stroke (Furie et al., 2011). Lp (a) was first described in 1963 and is an independent plasma lipoprotein; it includes an Apo B-containing LDL protein and Apo A. Previous studies have shown that Lp (a) is a risk factor for various diseases, including arterial ischemic stroke, coronary artery disease, intrauterine growth restriction, and certain cancers (Nagaraj et al., 2011; Nasr et al., 2011; Ashfaq et al., 2013; Goldenberg et al., 2013; Greif et al., 2013; Kronenberg and Utermann, 2013; Marrer et al., 2013; Suzuki et al., 2013; Gerhardt et al., 2014). Another study reported a positive association between vascular adhesion protein-1 (VAP-1) expression and Lp (a) (Nemati et al., 2013). However, the exact function and contribution of Lp (a) to cardiovascular disease are still unknown (Angelin, 2013).
In this study, we detected the MTHFR C677T polymorphism using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) in 174 patients with hypertension and 634 healthy controls from Jiangxi Province, China. We collected biochemical data to determine the blood lipid correlative index, including HCY, Lp (a), HDL, LDL, Apo AI, Apo B, GLU, TC, and TG, for association with the MTHFR C677T polymorphism and risk of hypertension.
Materials and Methods
Study population
A total of 174 patients with hypertension (blood pressure ≥140/90 mmHg) and 634 healthy control subjects from Jiangxi province, China, were enrolled in this study between June 2012 and September 2012. The mean age of the hypertension group was 68 years old (ranging from 28 to 90 years) and included 86 males and 88 females. The mean age of the healthy control group was 64 years old (ranging from 29 to 90 years) and included 313 males and 321 females. Individuals with any conditions, such as liver and kidney diseases, diabetes, autoimmune diseases or other malignant diseases, pregnancy or lactation, were excluded from the study. This study was approved by our hospital review board and each subject provided a written consent form before participation in this study. Five milliliters of venous blood was obtained from each individual after overnight fasting. The plasma was separated within an hour for biochemical parameter analyses, and white blood cells were collected for genotyping the MTHFR C677T polymorphism.
Genomic DNA extraction and PCR-RFLP analysis of the MTHFR genotype
Genomic DNA was extracted from white blood cells using the TIANamp Blood DNA Kit (Tiangen). The DNA concentration was measured with an ultraviolet spectrophotometer and then adjusted to 10 ng/μL with Tris/EDTA buffer. These DNA samples were stored at −20°C until use. For detection of the MTHFR C677T polymorphism, we performed PCR-RFLP using the following PCR primers: 5′-ATA AAT AAT AAA TAA AAT AAT AAA TAA ATA ATT ATT GGC AGG TTA CCC CAA A-3′ and 5′-CTC ACC TGG ATG GGA AAG AT-3′. PCR amplification was carried out in 20 μL PCR mixture in a PCR Amplifier (Long Gene) as follows: an initial denaturation at 94°C for 3 min and then 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 20 s, and a final extension at 72°C for 3 min. Next, the PCR products were digested by HinfI (Thermo) at 37°C for 1 h and then separated by 2% agarose gel electrophoresis stained with ethidium bromide (Fig. 1). Every sample was repeated at least once.
FIG. 1.
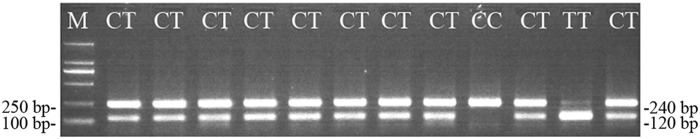
DNA gel electrophoresis after polymerase chain reaction (PCR) restriction products were digested by HinfI. The HinfI-digested PCR products were separated by 2% agarose gel electrophoresis and stained with ethidium bromide. The genotype TT product shows two 120 bp bands, whereas the genotype CC shows a 240 bp band. In contrast, the digested genotype CT shows 120 and 240 bp bands.
Analysis of blood lipid correlative index parameters
Plasma was separated from 5 mL of venous blood less than 1 h after being drawn and analyzed by an automatic biochemical analyzer (AU5400; Olympus) using commercially available kits (HCY kit was from AUSA; kits for Lp (a), HDL, LDL, Apo AI, Apo B, GLU, TC, and TG were from Biotech).
Statistical analyses
The SPSS 19.0 software (SPSS) was used for all statistical analyses. The blood lipid and correlative index parameters are expressed as the mean±standard deviation. The confidence intervals of the MTHFR allele and the genotype were computed according to a normal approximation with correction for continuity. The association between the blood lipid and correlative index parameters in the experiments and the controls was statistically analyzed using a Student t-test. The chi-square test was used to analyze the enumeration data. A p-value of <0.05 was considered to be statistically significant.
Results
Genotype and allele frequencies of the MTHFR C677T polymorphism between cases and controls
In this study, we detected the genotype and allele frequencies of the MTHFR C677T polymorphism in 174 patients with hypertension and 634 healthy control individuals (Table 1). There was a significant difference between the case and control groups (χ2=78.411, p<0.001 and χ2=57.319, p<0.001, respectively).
Table 1.
MTHFR C677T Genotype and Allele Frequencies in Case and Control Subjects
| MTHFR C677T genotype (%) | Allele frequency | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of persons | CC | CT | TT | 95% CI | C | T | 95% CI | |
| Case | 174 | 45 (25.9) | 53 (30.4) | 76 (43.7) | 51.1–36.3 | 41.1 | 58.9 | 64.1–53.7 |
| Control | 634 | 258 (40.7) | 291 (45.9) | 85 (13.4) | 10.9–16.1 | 63.6 | 36.4 | 33.8–39.0 |
The MTHFR 677TT genotype and 677T allele were significantly different between case and control subjects (χ2=78.411, p<0.001 and χ2=57.319, p<0.001, respectively).
MTHFR, methylene tetrahydrofolate reductase.
Blood lipid and correlative index between cases and controls
The biochemical characteristics of blood lipid and correlative indexes are shown in Table 2. Specifically, there were significant differences in HCY (p<0.001), TG (p=0.006), LDL (p=0.013), Apo-AI (p<0.001), and Lp (a) (p<0.001) levels between the hypertensive and control groups. In the hypertensive group, HCY (p=0.001), HDL (p=0.004), Apo AI (p<0.001), and TC (p=0.012) were significantly different between males and females, while differences in the GLU (p=0.174), Lp (a) (p=0.668), LDL (p=0.359), Apo B (p=0.095), and TG (p=0.073) levels were not statistically significant. In addition, there was a significant difference in HCY (p<0.001), HDL (p<0.001), Apo AI (p<0.001), GLU (p=0.001), and TC (p=0.004) levels, but not Lp (a) (p=0.789), LDL (p=0.278), Apo B (p=0.600), or TG (p=0.575) levels between males and females in the control group.
Table 2.
Average Blood Lipid Content and Correlative Indexes by Gender in Case and Control Subjects
| Male | 95% CI | Female | 95% CI | Total | 95% CI | |
|---|---|---|---|---|---|---|
| Case | ||||||
| HCY (μM)a | 27.27±8.60 | 25.63–29.31 | 23.50±6.02 | 22.29–24.81 | 25.36±7.62 | 24.25–26.55 |
| Lp (a) (g/L) | 37.71±20.21 | 33.66–42.20 | 34.37±21.89 | 30.16–39.15 | 36.02±25.14 | 32.97–39.59 |
| HDL (mM)a | 0.97±0.22 | 0.93–1.02 | 1.08±0.26 | 1.02–1.13 | 1.03±0.24 | 0.99–1.06 |
| LDL (mM) | 2.79±0.77 | 2.64–2.95 | 2.90±0.88 | 2.73–3.08 | 2.85±0.83 | 2.73–2.97 |
| Apo AI (g/L)b | 1.04±0.17 | 1.01–1.07 | 1.16±0.20 | 1.12–1.20 | 1.10±0.19 | 1.07–1.13 |
| Apo B (g/L) | 0.76±0.18 | 0.72–0.79 | 0.81±0.21 | 0.76–0.85 | 0.78±0.20 | 0.75–0.81 |
| GLU (mM) | 5.40±1.34 | 5.15–5.68 | 5.14±1.16 | 4.90–5.41 | 5.26±1.26 | 5.09–5.45 |
| TC (mM)c | 4.31±0.97 | 4.11–4.51 | 4.70±1.05 | 4.49–4.90 | 4.51±1.03 | 4.35–4.67 |
| TG (mM) | 1.59±1.144 | 1.36–1.83 | 1.99±1.77 | 1.66–2.38 | 1.79±1.50 | 1.58–2.02 |
| Control | ||||||
| HCY (μM)b | 19.79±9.96 | 18.76–21.00 | 14.82±7.70 | 14.02–15.69 | 17.27±9.22 | 16.60–18.00 |
| Lp (a) (g/L) | 19.63±15.54 | 17.96–21.55 | 19.29±16.65 | 17.45–21.13 | 19.46±16.10 | 18.25–20.70 |
| HDL (mM)b | 0.94±0.21 | 0.92–0.96 | 1.04±0.24 | 1.01–1.06 | 0.99±0.23 | 0.97–1.01 |
| LDL (mM) | 2.64±0.83 | 2.55–2.73 | 2.71±0.74 | 2.62–2.79 | 2.67±0.79 | 2.62–2.73 |
| Apo AI (g/L)b | 0.93±0.17 | 0.92–0.95 | 1.06±0.34 | 1.02–1.10 | 1.00±0.27 | 0.98–1.02 |
| Apo B (g/L) | 0.80±0.21 | 0.78–0.83 | 0.81±0.24 | 0.79–0.84 | 0.81±0.23 | 0.79–0.83 |
| GLU (mM)a | 5.07±1.32 | 4.94–5.21 | 5.55±2.13 | 5.31–5.79 | 5.31±1.79 | 5.18–5.46 |
| TC (mM)a | 4.30±1.01 | 4.19–4.41 | 4.52±0.94 | 4.41–4.62 | 4.41±0.98 | 4.34–4.49 |
| TG (mM) | 1.53±0.85 | 1.44–1.63 | 1.57±0.84 | 1.48–1.66 | 1.55±0.85 | 1.49–1.61 |
There were significant differences between males and females in HCY (p=0.001), HDL (p=0.004), Apo AI (p<0.001), and TC (p=0.012) levels among the case patients. There were also significant differences between males and females in HCY (p<0.001), HDL (p<0.001), Apo AI (p<0.001), GLU (p=0.001), and TC (p=0.004) among the control subjects.
p<0.01, bp<0.001, cp<0.05.
Apo AI, apolipoprotein AI; GLU, glucose; HCY, homocysteine; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Lp (a), lipoprotein (a); TC, total cholesterol; TG, triglyceride.
Associations of the MTHFR C677T polymorphism with blood biochemical data in cases versus controls
We then combined MTHFR C677T polymorphisms with blood biochemical data from case and control subjects for analysis. We found that the average HCY and Lp (a) levels were significantly higher in patients with a MTHFR 677CC/CT or 677TT genotype in the hypertensive group than controls (pHCY<0.001, pLp (a)<0.001) (Table 3). In the hypertension group, HCY and Lp (a) levels in patients with the TT genotype were significant higher than in patients with a CC/CT genotype (pHCY<0.001, pLp (a)=0.003). There were similar findings in the control group (pHCY<0.001, pLp (a)=0.005).
Table 3.
Levels of Blood Lipids and Correlated Indexes by Gene Polymorphism Between Case and Control Subjects
| Case | Control | |||
|---|---|---|---|---|
| CC+CT | TT | CC+CT | TT | |
| HCY (μM) | 26.58±8.36 | 29.69±7.75 | 16.48±7.79 | 22.41±14.61 |
| Lp (a) (g/L) | 31.55±19.12 | 42.50±19.22 | 18.78±14.99 | 24.00±21.53 |
| HDL (mM) | 0.98±0.22 | 1.01±0.24 | 0.99±0.23 | 0.98±0.23 |
| LDL (mM) | 2.81±0.77 | 2.91±0.86 | 2.65±0.76 | 2.83±0.92 |
| Apo AI (g/L) | 1.05±0.17 | 1.09±0.20 | 1.00±0.28 | 0.99±0.20 |
| Apo B (g/L) | 0.76±0.18 | 0.79±0.21 | 0.81±0.23 | 0.83±0.21 |
| GLU (mM) | 5.39±1.29 | 5.45±1.50 | 5.30±1.77 | 5.35±1.92 |
| TC (mM) | 4.39±0.99 | 4.50±1.13 | 4.39±0.95 | 4.57±1.14 |
| TG (mM) | 1.68±1.24 | 1.73±1.55 | 1.55±0.85 | 1.55±0.79 |
HCY and Lp (a) levels in those with the TT genotype were significantly higher than in those with the CC/CT genotype, regardless of whether they were case or control subjects. HCY: p<0.001, Lp (a): control, p=0.005; hypertensive, p=0.003.
As shown in Table 4, we divided the hypertensive and control groups by gender. We discovered that only the HCY level in patients with a TT genotype was significantly higher than in patients with a CC/CT genotype (pHCY<0.001) between males and females in both the hypertensive and control groups.
Table 4.
Levels of Blood Lipids and Correlated Indexes by Gene Polymorphism and Gender in Case and Control Subjects
| Case | Control | |||
|---|---|---|---|---|
| CC+CT | TT | CC+CT | TT | |
| Male | ||||
| HCY (μM)a | 23.62±6.53 | 30.29±8.99 | 18.78±8.74 | 26.68±14.30 |
| Lp (a) (g/L) | 30.15±15.32 | 43.99±21.72 | 18.96±14.55 | 24.20±20.72 |
| HDL (mM) | 0.99±0.21 | 0.96±0.23 | 0.94±0.21 | 0.94±0.20 |
| LDL (mM) | 2.80±0.74 | 2.78±0.79 | 2.60±0.78 | 2.92±1.08 |
| Apo AI (g/L) | 1.05±0.17 | 1.03±0.18 | 0.94±0.17 | 0.93±0.15 |
| Apo B (g/L) | 1.05±0.17 | 1.03±0.18 | 0.79±0.21 | 0.86±0.24 |
| GLU (mM) | 5.33±1.20 | 5.45±1.46 | 5.06±1.33 | 5.08±1.21 |
| TC (mM) | 4.36±0.97 | 4.27±0.98 | 4.25±0.96 | 4.63±1.25 |
| TG (mM) | 1.54±0.99 | 1.62±1.26 | 1.53±0.86 | 1.55±0.82 |
| Female | ||||
| HCY (μM)a | 20.94±4.60 | 28.70±5.17 | 14.20±5.91 | 18.62±13.96 |
| Lp (a) (g/L) | 32.46±24.67 | 38.26±14.30 | 18.55±15.43 | 23.81±22.45 |
| HDL (mM) | 1.07±0.27 | 1.09±0.23 | 1.04±0.23 | 1.02±0.26 |
| LDL (mM) | 2.80±0.84 | 3.11±0.95 | 2.70±0.74 | 2.75±0.76 |
| Apo AI (g/L) | 1.14±0.20 | 1.19±0.18 | 1.06±0.35 | 1.03±0.23 |
| Apo B (g/L) | 0.78±0.19 | 0.85±0.25 | 0.81±0.25 | 0.81±0.17 |
| GLU (mM) | 4.98±0.85 | 5.45±1.59 | 5.54±2.09 | 5.59±2.38 |
| TC (mM) | 4.61±0.93 | 4.87±1.26 | 4.52±0.92 | 4.51±1.05 |
| TG (mM) | 2.03±1.69 | 1.92±1.95 | 1.57±0.86 | 1.56±0.77 |
The HCY levels with the TT genotype were significantly higher than with the CC/CT genotype in both case and control subjects.
p<0.001.
Discussion
Due to variable genetic polymorphisms, geographic residencies, and lifestyle, an individual's predisposition and susceptibility to hypertension may vary, especially in China (Yang et al., 2013). There have been no reports regarding the MTHFR C677T polymorphism and hypertension in Jiangxi, China. Thus, in this study, we compared the rate of the 677TT genotype and the T allele between hypertensive and control subjects in this region. The genotype rate was significantly higher in the hypertensive group than in the healthy control group, suggesting that the MTHFR C677T gene polymorphism may be a risk factor for hypertension. These findings are consistent with some similar studies in other geographical locations (Marinho et al., 2007; Alghasham et al., 2012). However, it is different than other gene polymorphisms. For example, the eNOS T786C polymorphism is associated with hypertension risk in Beijing but not in Jilin (Li et al., 2011).
The MTHFR protein is an important enzyme in HCY metabolism (Huang et al., 2012). A 677C→T mutation results in a switch from alanine to valine. This mutation leads to decreased MTHFR enzymatic activity and increases the enzyme's sensitivity to heat (Zonouzi et al., 2012). Thus, MTHFR gene mutations that are associated with higher levels of HCY are considered to be risk factors for cardiovascular disease (Yin et al., 2012; Kevere et al., 2013; Xu et al., 2013). However, there is controversy about the relationship between the mutation and the rise in HCY levels. Our studies show that the MTHFR C677T mutation alters the serum level of HCY, which is consistent with some previous reports (Zong et al., 2011; Zhang et al., 2012; Nienaber-Rousseau et al., 2013; Bharatkumar et al., 2014) but contrasts with other studies (Yasar et al., 2012; Creus et al., 2013; de Carvalho et al., 2013). Our results indicate that there are variations in the HCY levels among various MTHFR677 genotypes. In this study, the HCY level and the frequency of the TT genotype in the hypertensive group were both significantly higher than in the control group. Thus, the MTHFR C677T polymorphisms may be a risk factor for hypertension through increased HCY levels.
Studies have indicated a correlation between the MTHFR C677T polymorphism and the Lp (a) level in coronary artery disease (Balogh et al., 2012) as well as in unexplained recurrent miscarriages (Krause et al., 2005) and other diseases. However, few studies have analyzed the relationship between the MTHFR C677T polymorphism and Lp (a). MTHFR may indirectly affect Lp (a) metabolism. Therefore, further studies using a larger population are necessary to confirm our discovery.
In summary, this study showed significant differences in the MTHFR C677T polymorphism distribution in Chinese hypertensive patients. In addition, the MTHFR C677T polymorphism may be a risk factor for hypertension. Our findings may be helpful for designing more effective treatment measures for hypertension patients in specific populations. Furthermore, HCY levels are likely associated with the MTHFR C677T polymorphism and gender. In addition, we found that the Lp (a) level is separated from the genotype polymorphism, which has rarely been reported in previous studies.
Acknowledgments
This work was supported, in part, by grants from the National Natural Science Foundation of China (No. 81271912 and No. 81360032).
Author Disclosure Statement
All the authors declare that they have no conflicts of interest, financial or otherwise, in the publication of this study or its results.
References
- Ala AL, Tim A, Douglas B, et al. (2011) Global status report on noncommunicable diseases. Available at www.who.int/nmh/publications/ncd_report2010/en/, accessed at April2011
- Alghasham A, Settin AA, Ali A, et al. (2012) Association of MTHFR C677T and A1298C gene polymorphisms with hypertension. Int J Health Sci (Qassim) 6:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin B. (2013) Fifty years of lipoprotein(a)—the magical mystery tour continues. J Intern Med 273:3–5 [DOI] [PubMed] [Google Scholar]
- Ashfaq F, Goel PK, Sethi R, et al. (2013) Lipoprotein (a) levels in relation to severity of coronary artery disease in North Indian patients. Heart Views 14:12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh E, Bereczky Z, Katona E, et al. (2012) Interaction between homocysteine and lipoprotein(a) increases the prevalence of coronary artery disease/myocardial infarction in women: a case-control study. Thromb Res 129:133–138 [DOI] [PubMed] [Google Scholar]
- Bayramoglu A, Urhan Kucuk M, Guler HI, et al. (2013) Is there any genetic predisposition of MMP-9 gene C1562T and MTHFR gene C677T polymorphisms with essential hypertension? Cytotechnology [Epub ahead of print]; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharatkumar VP, Nagaraja D, Christopher R. (2014) Hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T polymorphism in cerebral veno-sinus thrombosis. Clin Appl Thromb Hemost 20:78–83 [DOI] [PubMed] [Google Scholar]
- Creus M, Deulofeu R, Penarrubia J, et al. (2013) Plasma homocysteine and vitamin B12 serum levels, red blood cell folate concentrations, C677T methylenetetrahydrofolate reductase gene mutation and risk of recurrent miscarriage: a case-control study in Spain. Clin Chem Lab Med 51:693–699 [DOI] [PubMed] [Google Scholar]
- Davis LA, Cannon GW, Pointer LF, et al. (2013) Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol 40:809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho SC, Muniz MT, Siqueira MD, et al. (2013) Plasmatic higher levels of homocysteine in Non-alcoholic fatty liver disease (NAFLD). Nutr J 12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie KL, Kasner SE, Adams RJ, et al. (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 42:1–36 [DOI] [PubMed] [Google Scholar]
- Gerhardt A, Howe N, Krussel JS, et al. (2014) Elevated lipoprotein(a) levels and homozygous human platelet antigen 1b (HPA-1b) genotype are risk factors for intrauterine growth restriction (IUGR). J Thromb Thrombolysis 37:107–117 [DOI] [PubMed] [Google Scholar]
- Goldenberg NA, Bernard TJ, Hillhouse J, et al. (2013) Elevated lipoprotein (a), small apolipoprotein (a), and the risk of arterial ischemic stroke in North American children. Haematologica 98:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif M, Arnoldt T, von Ziegler F, et al. (2013) Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med 24:75–79 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Kotwal J, Kotwal A, et al. (2012) Role of homocysteine & MTHFR C677T gene polymorphism as risk factors for coronary artery disease in young Indians. Indian J Med Res 135:506–512 [PMC free article] [PubMed] [Google Scholar]
- Hankey GJ, Eikelboom JW. (1999) Homocysteine and vascular disease. Lancet 354:407–413 [DOI] [PubMed] [Google Scholar]
- Huang T, Wahlqvist ML, Li D. (2012) Effect of n-3 polyunsaturated fatty acid on gene expression of the critical enzymes involved in homocysteine metabolism. Nutr J 11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Zhao X, Liu L, et al. (2013) Association of the C677T polymorphism in the MTHFR gene with hemorrhagic stroke: a meta-analysis. Genet Test Mol Biomarkers 17:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevere L, Purvina S, Bauze D, et al. (2013) Homocysteine and MTHFR C677T polymorphism in children and adolescents with psychotic and mood disorders. Nord J Psychiatry 6:1271–1275 [DOI] [PubMed] [Google Scholar]
- Krause M, Sonntag B, Klamroth R, et al. (2005) Lipoprotein (a) and other prothrombotic risk factors in Caucasian women with unexplained recurrent miscarriage. Results of a multicentre case-control study. Thromb Haemost 93:867–871 [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Utermann G. (2013) Lipoprotein(a): resurrected by genetics. J Intern Med 273:6–30 [DOI] [PubMed] [Google Scholar]
- Li J, Cun Y, Tang WR, et al. (2011) Association of eNOS gene polymorphisms with essential hypertension in the Han population in southwestern China. Genet Mol Res 10:2202–2212 [DOI] [PubMed] [Google Scholar]
- Malinowska J, Kolodziejczyk J, Olas B. (2012) The disturbance of hemostasis induced by hyperhomocysteinemia; the role of antioxidants. Acta Biochim Pol 59:185–194 [PubMed] [Google Scholar]
- Marinho C, Alho I, Arduino D, et al. (2007) GST M1/T1 and MTHFR polymorphisms as risk factors for hypertension. Biochem Biophys Res Commun 353:344–350 [DOI] [PubMed] [Google Scholar]
- Marrer E, Wagner A, Montaye M, et al. (2013) Lipoprotein(a) plasma levels and the risk of cancer: the PRIME study. Eur J Cancer Prev 22:286–293 [DOI] [PubMed] [Google Scholar]
- Nagaraj SK, Pai P, Bhat G, et al. (2011) Lipoprotein (a) and other lipid profile in patients with thrombotic stroke: is it a reliable marker? J Lab Physicians 3:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr N, Ruidavets JB, Farghali A, et al. (2011) Lipoprotein (a) and carotid atherosclerosis in young patients with stroke. Stroke 42:3616–3618 [DOI] [PubMed] [Google Scholar]
- Nemati H, Khodarahmi R, Rahmani A, et al. (2013) Serum lipid profile in psoriatic patients: correlation between vascular adhesion protein 1 and lipoprotein (a). Cell Biochem Funct 31:36–40 [DOI] [PubMed] [Google Scholar]
- Nienaber-Rousseau C, Pisa PT, Venter CS, et al. (2013) Nutritional genetics: the case of alcohol and the MTHFR C677T polymorphism in relation to homocysteine in a Black South African population. J Nutrigenet Nutrigenomics 6:61–72 [DOI] [PubMed] [Google Scholar]
- Smulders YM, Blom HJ. (2011) The homocysteine controversy. J Inherit Metab Dis 34:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Futami-Suda S, Igari Y, et al. (2013) Low-molecular-weight lipoprotein (a) and low relative lymphocyte concentration are significant and independent risk factors for coronary heart disease in patients with type 2 diabetes mellitus: Lp(a) phenotype, lymphocyte, and coronary heart disease. Lipids Health Dis 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taioli E, Garza MA, Ahn YO, et al. (2009) Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: a HuGE-GSEC review. Am J Epidemiol 170: 1207–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang Y, Gong F, et al. (2013) MTHFR C677T polymorphism and risk of congenital heart defects: evidence from 29 case-control and TDT studies. PLoS One 8:e58041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch GN, Loscalzo J. (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050 [DOI] [PubMed] [Google Scholar]
- Wilson CP, McNulty H, Scott JM, et al. (2010) Postgraduate symposium: the MTHFR C677T polymorphism, B-vitamins and blood pressure. Proc Nutr Soc 69:156–165 [DOI] [PubMed] [Google Scholar]
- Xu H, Liu C, Wang Q. (2013) Plaque image characteristics, hyperhomocysteinemia, and gene polymorphism of homocysteine metabolism-related enzyme (MTHFR C677T) in acute coronary syndrome. Cell Biochem Biophys 66:403–407 [DOI] [PubMed] [Google Scholar]
- Yang B, Liu Y, Li Y, et al. (2013) Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS One 8:e57917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar A, Gunduz K, Onur E, et al. (2012) Serum homocysteine, vitamin B12, folic acid levels and methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in vitiligo. Dis Markers 33: 85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Yan L, Zhang Z, et al. (2012) C677T methylenetetrahydrofolate reductase gene polymorphism as a risk factor involved in venous thromboembolism: a population-based case-control study. Mol Med Rep 6:1271–1275 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Lu YQ, Li HF, et al. (2012) Methylenetetrahydrofolate reductase and methionine synthase reductase gene polymorphisms in ethnic Han women from Linyi. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 29:705–708 [DOI] [PubMed] [Google Scholar]
- Zhao X, Jiang H. (2013) Quantitative assessment of the association between MTHFR C677T polymorphism and hemorrhagic stroke risk. Mol Biol Rep 40:573–578 [DOI] [PubMed] [Google Scholar]
- Zong Y-H, Xu X-P, Huo Y, et al. . Genetic and environmental factors for H-type hypertension in Anqing rural community. Chin J Hypertens 19:2011 [Google Scholar]
- Zonouzi AP, Nader Chaparzadeh, Estiar MA. (2012) Methylene tetrahydrofolate reductase C677T and A1298C mutations in women with recurrent spontaneous abortions in the Nothwest of Iran. Obstet Gynecol 2012:Article ID 945486 [DOI] [PMC free article] [PubMed] [Google Scholar]


