Abstract
The composition of the human intestinal flora is important for the health status of the host. The global composition and the presence of specific pathogens are relevant to the effects of the flora. Therefore, accurate quantification of all major bacterial populations of the enteric flora is needed. A TaqMan real-time PCR-based method for the quantification of 20 dominant bacterial species and groups of the intestinal flora has been established on the basis of 16S ribosomal DNA taxonomy. A PCR with conserved primers was used for all reactions. In each real-time PCR, a universal probe for quantification of total bacteria and a specific probe for the species in question were included. PCR with conserved primers and the universal probe for total bacteria allowed relative and absolute quantification. Minor groove binder probes increased the sensitivity of the assays 10- to 100-fold. The method was evaluated by cross-reaction experiments and quantification of bacteria in complex clinical samples from healthy patients. A sensitivity of 101 to 103 bacterial cells per sample was achieved. No significant cross-reaction was observed. The real-time PCR assays presented may facilitate understanding of the intestinal bacterial flora through a normalized global estimation of the major contributing species.
The bacterial inhabitants of the human gastrointestinal tract constitute a complex ecosystem that includes both aerobic and anaerobic microorganisms. Four hundred to five hundred bacterial species are estimated to be present in the human fecal flora at concentrations of up to 1012 viable microorganisms per g of stool (29). The total number of bacterial cells of the intestinal flora is estimated to approximately 1014. According to conventional culture, the indigenous flora is relatively stable and consists of the four major bacterial groups Bacteroides, Bifidobacterium, Eubacterium, and Peptostreptococcus at concentrations of approximately 1010 to 1011 CFU/g, also called the dominant flora. The subdominant flora consists of bacteria belonging to the genera Streptococcus, Lactobacillus, and, to a lesser extent, Enterococcus, Clostridium, Bacillus, and yeasts at concentrations of 106 to 108 CFU/g.
The intestinal bacterial flora shows important interactions with the host. These interactions are currently poorly understood, but a number of findings indicate an influence on the health status of the host. In inflammatory bowel diseases, intestinal mucosal inflammation emerges from abnormal immune reactivity to altered enteric bacterial flora (1, 6, 25, 26). Genetic predisposition to disease, such as the recently described NOD2/CARD15 gene variants, may relate to disturbed bacterial recognition (11, 14, 24). Other intestinal diseases, such as infectious diarrhea in children and irritable bowel syndrome, benefit from probiotic treatment, supporting the assumption that an altered intestinal flora may contribute to these diseases (28, 31, 32, 33). Recent studies propose a role for intestinal bacterial flora in the pathogenesis and pathophysiology of a number of extraintestinal diseases. In infants with atopic disease, differences in the composition of the intestinal microflora were found long before the development of any clinical manifestations (3, 16). The health effects of the flora are most probably not mediated by specific, single species but rather by the global composition (2). Therefore, quantification of the global composition is a critical requirement for understanding the health effects of the intestinal flora.
Culture is the classical approach for the identification and quantification of bacteria. Most of the data available on the gut bacteria have been generated by cultivation and enumeration (29). Though selective growth media and special growth conditions have been developed to culture intestinal bacteria, in complex bacterial communities only a small part, 10% to 40%, of the flora is covered (18, 22, 36). The 16S ribosomal DNA (rDNA) is a suitable marker gene for taxonomic and phylogenetic applications (1, 8, 20, 30, 37, 38). Real-time PCR with species-specific probes can provide an accurate and sensitive method for quantification of individual species and bacterial populations as well as total bacteria (4, 10, 13, 21, 27). Real-time PCR has been shown to provide a linear range of detection from 10 to more than 108 cells (12). For quantitative purposes, real-time PCR is more reliable than other methods such as single-strand conformation polymorphism analysis, temperature gradient gel electrophoresis, and fluorescence in situ hybridization (5, 23).
The use of individual specific primer and probe combinations represents an established method to quantify individual bacteria. Real-time PCR has been used successfully to quantify specific bacterial species from the intestinal mucosa and stool (7, 15, 23). As outlined above, understanding the global composition of the flora is critical to understanding its function. Therefore, we aimed for a method combining accurate quantification and global estimation of all major bacterial species. We decided to implement this approach through a modified real-time method. This method combines a pair of conserved amplification primers with universal and specific (species, group, and genus specific) quantification probes in a single reaction. Implementation has been significantly facilitated by the use of oligonucleotide probes with conjugated minor groove binders (MGBs), which form extremely stable duplexes with the target DNA and provide a sharper binding profile in the PCR than conventional oligonucleotide probes (17). We demonstrate the feasibility of the method for 20 major gastrointestinal bacterial probes and in the practical application to clinical samples.
MATERIALS AND METHODS
Biopsy specimens.
Biopsy specimens for real-time experiments were taken from the lower part of the colon (sigma) during routine endoscopy. Antimicrobial therapy within the previous 6 months was ruled out by history. Patients gave written informed consent prior to endoscopy. The study was approved by the local ethics committee. The biopsy samples were snap frozen in liquid nitrogen immediately after endoscopy.
Extraction of DNA from biopsy and stool samples.
Biopsies were first incubated with 200 μl of TL buffer and 25 μl of protease K (PqLab, Erlangen, Germany) at 55°C for 2 h to disrupt cell walls. DNA of biopsy and stool samples was isolated with the FastDNA spin kit for soil (Bio 101) after mechanical homogenization (FastPrep FP 120 instrument; Bio 101) according to the manufacturer's instructions. The DNA was checked by 1.5% agarose gel electrophoresis, and the DNA amount was measured by photometry at 260 and 280 nm.
Bacterial strains, cloned plasmid DNA, and generation of standard curves.
Escherichia coli (ATCC 25922), Bacteroides fragilis (ATCC 25285), Bacteroides thetaiotaomicron (ATCC 29742), Bifidobacterium adolescentis (DSM 20083), Bifidobacterium bifidum (ATCC 29521), Bifidobacterium longum (DSM 20219), Campylobacter faecalis (NCTC 11415), Clostridium difficile (laboratory isolate, Hospital of Grosshaden, Munich, Germany), Enterobacter cloacae (DSM 13047), Enterococcus durans (NCTC 8307), Enterococcus faecalis (NCTC 775), Eubacterium lentum (ATCC 43055), Fusobacterium nucleatum (ATCC 10953), Helicobacter pylori (laboratory isolate), Klebsiella pneumoniae (DSM 13882), Lactobacillus acidophilus (NCTC 1723), Peptostreptococcus anaerobius (laboratory isolate, University of Rostock, Germany), Peptostreptococcus productus (ATCC 27340), Proteus vulgaris (ATCC13315), Salmonella enteritidis (laboratory isolate), Staphylococcus aureus (ATCC 25923), and Streptococcus faecium (DSM 2146) were kindly provided by U. Ullmann (Institute for Medical Microbiology and Virology, University of Kiel, Kiel, Germany). 16S rDNAs from Megasphaera elsdenii, Ruminococcus hansenii, Ruminococcus albus, Fusobacterium sp., Clostridium xylanolyticum, Clostridium symbiosum, Lactococcus lactis, Eubacterium ramulus, Streptococcus parasanguis, and Streptococcus salivarius were obtained from cloned 16S rDNA libraries.
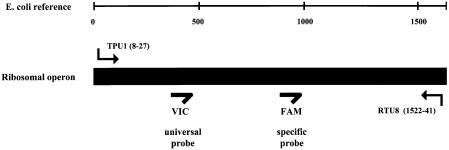
The initial PCR for the clone libraries was performed with forward primer TPU1 (AGAGTTTGATCMTGGCTCAG, positions 8 to 27) and reverse primer RTU8 (AAGGAGGTGATCCANCCRCA, positions 1522 to 41, Escherichia coli reference numbering). DNA was cloned into competent Escherichia coli cells with the pCR2.1 TOPO TA cloning kit (Invitrogen, Karlsruhe, Germany). Plasmid DNA from overnight cultures was prepared with the QIAprep 96 Turbo miniprep kit (Qiagen, Hilden, Germany). Sequencing of the inserts was performed on an ABI Prism 3700 DNA Analyzer in a final volume of 10 μl with 1 μl of ABI Prism BigDye (Applied Biosystems) and a 1.6 μM concentration of each primer with the following protocol: 96°C for 3 min and 25 cycles of 95°C for 40 s, 55°C for 40 s, and 60° for 4 min.
The bacteria used for the quantification process were cultivated on selective media under either aerobic or anaerobic conditions. The 16S rDNA of each strain was sequenced as described below to ensure that bacteria grown on different media were consistent with the bacterial strains originally used for cultivation. The total number of cells (i.e., the number of CFU) was enumerated with a Neubauer chamber several times independently by two persons. Tenfold serial dilutions of the bacterial suspension were made, and the resulting dilutions were independently enumerated again in the same way by two persons. To generate a standard curve for real-time PCR, the bacterial DNA was extracted from the different dilutions and the concentration was adjusted; 1 μl of DNA thus corresponded to a defined number of CFU (100 to 108). The CT values at the different dilution points were averaged. The total number of cells was interpolated from the averaged standard curve as described elsewhere (21).
Isolation of DNA from bacterial cultures.
After counting the reference bacteria in different dilutions in Neubauer chambers, the cells were centrifuged (8,000 × g, 10 min at room temperature) and frozen at −20°C. DNA was isolated from individual bacterial species after mechanical homogenization (FastPrep FP 120 instrument; Bio 101) with the FastDNA spin kit for soil (Bio 101) according to the manufacturer's instructions. The DNA was checked on 1.5% agarose gels and measured by photometry at 260 and 280 nm.
Probe design for real-time PCR.
Fluorescently labeled oligonucleotide probes were designed with the Probe Design tool of the ARB software package that is available on the Internet (W. Ludwig, Department of Microbiology, Technical University, Munich, Germany; http://www.arb-home.de/, accessed as of January 2002). The ARB software is a graphically oriented software package comprising various tools for 16S rDNA sequence database handling and data analysis. The probe design tool of ARB software provides specificity for species-, group-, and genus-specific probes. The MGB probes were tested for their oligonucleotide parameters with Primer Express software version 2.0 (Applied Biosystems). Probes were checked for specificity and cross-reactivity with the Probe Match tool of the ARB software package. For additional confidence, probes were aligned with 16S sequences from public databases.
Cross-reaction experiments with closely related species were performed to rule out relevant cross-reactions. Briefly, the species- and group-specific probes were tested with DNA from closely related bacterial species as far as available to ensure specificity. No relevant (CT > 40) overlap in the fluorescence signal was detected between the different probes. Defined amounts of DNA from a known species were quantified by real-time PCR and additionally spiked in a mixture of DNA from other bacterial strains or DNA extracted from samples. The fluorescence signal given by the DNA alone was then compared to the signal obtained from the spiked DNA. No significant differences were observed, indicating high specificity of the probes used and no relevant cross-reaction.
Real-time PCR.
Amplification and detection were carried out in 96-well optical plates on an ABI Prism 7700 sequence detector with TaqMan Universal PCR 2x master mix (Applied Biosystems), a 0.4 μM concentration of each primer, a 0.2 μM concentration of each probe, and 1 to 200 ng of sample DNA in a final volume of 20 μl per reaction. The whole 16S rDNA sequence was amplified for quantitative PCR with an initial hold of 50°C for 2 min to activate the No Amp Erase UNG and a hold of 95°C for 10 min to activate AmpliTaq Gold polymerase, followed by 50 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 3 min. The specific fluorescent probes were labeled at the 5′end with the reporter dye 6-carboxyfluorescin (FAM); the universal probe is labeled with 6-carboxyrhodamine (VIC). In the present study, MGB fluorescent probes with nonfluorescent quencher dyes (also called dark quenchers) were used (Applied Biosystems). The real-time PCR experiments were performed on an ABI 7700 with the software upgrade for nonfluorescent quencher probe support. The primers used in this study hybridize to conserved regions on the 16S rRNA gene. The forward primer TPU1 (AGAGTTTGATCMTGGCTCAG) binds to positions 8 to 27, and the reverse primer RTU8 (AAGGAGGTGATCCANCCRCA) binds to positions 1522 to 41 (Escherichia coli reference numbering).
RESULTS
Comparison of MGB probes and normal fluorescent TaqMan probes.
To increase the sensitivity of quantification and to minimize optimization of real-time PCR assays, MGB fluorescent probes were used. MGB probes and probes without minor groove binder molecules of the same sequence were compared under the same reaction conditions. A serial dilution of bacterial DNA of Escherichia coli, Enterococcus durans, and Bacteroides fragilis was measured with the VIC-labeled universal probe and the FAM-labeled specific probes. A conventional nonquantitative PCR was performed in parallel. The CT values and the related cell numbers were determined in real-time PCR. The recorded gradual increase in the samples' fluorescence above an established baseline value is proportional to the amount of the accumulated PCR product up to this point. The baseline value is established during the initial cycles, when there is only an insignificant change in the total sample fluorescence. As the PCR progresses into the exponential phase, the system detects a cycle when the fluorescence detected is significantly higher than the baseline value. This point is defined as a threshold cycle (CT). The CT values of each real-time PCR depend on the initial template amount (copy number) of the target sequence and are inversely proportional to the log of this copy number.
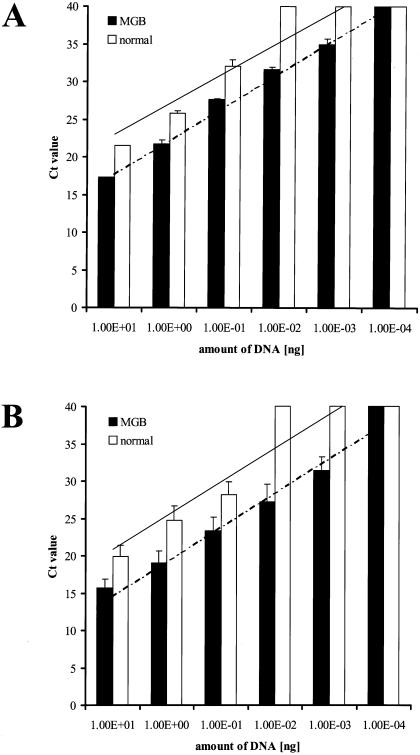
As shown in Table 1, the detection limits for the universal probe and the specific MGB probes for Escherichia coli and Enterococcus durans were 2 log ranks higher than for the probes without a minor groove binder. For Bacteroides fragilis, the detection limit with the specific MGB probe was 1 log rank higher than that with the normal probe. Figure 1 shows the standard curves for the VIC-labeled universal probe and the averaged standard curves for the FAM-labeled specific probes. TaqMan quantification demonstrated similar slopes of detection with MGB and non-MGB probes (4.49 versus 4.09 for the universal probe and 4.63 versus 4.52 for the specific probes) but different axis intercepts (13.17 versus 18.91 for the universal probe and 9.89 versus 16.32 for the specific probes).
TABLE 1.
Detection of serial dilutions of bacterial DNA: comparison of MGB and non-MGB probes
| Probe and DNA concn (label) |
CTa with probe:
|
Detection by conventional PCR with probe:
|
||
|---|---|---|---|---|
| MGB | Non-MGB | MGB | Non-MGB | |
| Universal probe (VIC) | ||||
| 10−4 | ≥40 | ≥40 | + | + |
| 10−3 | 34.990 ± 0.71 | ≥40 | + | + |
| 10−2 | 31.560 ± 0.33 | ≥40 | + | + |
| 10−1 | 27.585 ± 0.21 | 32.020 ± 0.92 | + | + |
| 100 | 21.755 ± 0.50 | 25.815 ± 0.28 | + | + |
| 101 | 17.335 ± 0.01 | 21.490 ± 0.06 | + | + |
| Escherichia coli (FAM) | ||||
| 10−4 | ≥40 | ≥40 | + | + |
| 10−3 | 31.330 ± 1.78 | ≥40 | + | + |
| 10−2 | 28.100 ± 0.17 | ≥40 | + | + |
| 10−1 | 24.760 ± 2.01 | 26.263 ± 0.46 | + | + |
| 100 | 20.715 ± 0.18 | 24.878 ± 0.19 | + | + |
| 101 | 16.925 ± 0.28 | 21.043 ± 0.29 | + | + |
| Enterococcus durans (FAM) | ||||
| 10−4 | ≥40 | ≥40 | + | + |
| 10−3 | 29.530 ± 1.03 | ≥40 | + | + |
| 10−2 | 24.455 ± 2.01 | ≥40 | + | + |
| 10−1 | 21.265 ± 0.05 | 29.417 ± 2.41 | + | + |
| 100 | 17.390 ± 0.13 | 26.628 ± 2.48 | + | + |
| 101 | 14.660 ± 0.13 | 20.457 ± 0.56 | + | + |
| Bacteroides fragilis (FAM) | ||||
| 10−4 | ≥40 | ≥40 | + | + |
| 10−3 | 33.185 ± 0.23 | ≥40 | + | + |
| 10−2 | 28.930 ± 0.18 | 34.257 ± 1.90 | + | + |
| 10−1 | 23.985 ± 0.57 | 28.937 ± 0.07 | + | + |
| 100 | 18.915 ± 0.23 | 22.767 ± 0.42 | + | + |
| 101 | 15.650 ± 1.58 | 18.084 ± 0.50 | + | + |
Mean value of two independent experiments ± standard deviation.
FIG. 1.
Comparison of MGB probes and probes without a minor groove binder in histograms of CT values of serial dilutions of bacterial DNA. (A) Dilution series of DNA from Escherichia coli detected with the VIC-labeled universal probe. MGB probes are more sensitive than probes without a minor groove binder (detection limit, 10−3 versus 10−1 ng of DNA). The trend lines show similar slopes (MGB probe, 4.49; normal probe, 4.09) but different axis intercepts (MGB probe, 13.17; normal probe, 18.91). (B) Mean CT values of a dilution series of DNA from Escherichia coli, Enterococcus durans, and Bacteroides fragilis, detected with FAM-labeled specific probes. The sensitivity of real-time PCR was higher with MGB probes than with probes without a minor groove binder (10−3 versus 10−1 ng of DNA). The trend lines show similar slopes (MGB probe, 4.52; normal probe, 4.63) but different axis intercepts (MGB probe, 9.89; normal probe, 16.32).
Sensitivity and specificity of the real-time PCR assay.
Cross-reaction experiments with DNA of the bacterial strains and cloned plasmid 16S rDNA as described above were performed. No significant cross-reaction of specific probes between the different species and bacterial groups was seen (data not shown). The sensitivity of detection was assessed by serial dilution experiments. Most of the specific FAM-labeled probes showed a sensitivity of ≈101 cells (Table 2). The limit of detection for some of the specific probes was ≈103 cells (Eubacterium lentum, Helicobacter pylori, Staphylococcaceae, and Streptococcaceae). The spiking experiments were performed to test the ability of this assay to pick out a specific bacterial DNA from a background of a complex DNA sample (i.e., extracted from biopsy specimens and stool samples).
TABLE 2.
Characteristics of molecular probes used in this study
| Groupa | Positionsb | Tm (°C) | Sequence | Source or reference | Detection limit | Dye |
|---|---|---|---|---|---|---|
| All bacteria | 321-337 | 66.4 | ACTGAGACACGGTCCA | 35 | 100 | VIC |
| Echerichia, Salmonella | 836-849 | 65.8 | GTGCCCTTGAGGC | 35 | 101 | FAM |
| Bacteroides fragilis, B. uniformis, B. thetaiotaomicron, B. ovatus, B. eggerthii, B. acidifaciens | 1159-1176 | 66.0 | TCACATCTTACGACGGC | ARB | 101 | FAM |
| Bacteroides, Porphyromonas, Prevotella | 1081-1097 | 66.8 | CACTTAGCCGACACCT | ARB | 101 | FAM |
| Clostridium difficile | 208-223 | 64.4 | ATCCTGTACTGGCTC | ARB | 101 | FAM |
| Clostridium xylanolyticum et rel. (C. sphenoides, C. celerecrescens, C. metoxy-benzovorans, C. aerotolerans, C. xylanolyticum, C. guttoideum, C. desulfotomaculum) | 992-1008 | 69.2 | CGGTCATTCCGATGTC | ARB | 101 | FAM |
| Clostridium symbiosum et rel. (C. clostridiiformes, C. symbiosum) | 646-662 | 67.3 | CCGACACTCTAGCAAA | ARB | 101 | FAM |
| Enterococcaceae | 1258-1274 | 67.2 | CTTAGCCTCGCGACTT | ARB | 101 | FAM |
| Enterobacteriaceae | 1418-1432 | 63.2 | CTTTTGCAACCCACT | ARB | 101 | FAM |
| Eubacterium lentum | 194-207 | 67.7 | AGCCCAGACGGCA | ARB | 103 | FAM |
| Fusobacterium | 746-763 | 67.3 | CTTTAGCGTCAGTATCT | ARB | 101 | FAM |
| Lactobacillales | 455-471 | 66.0 | AGGCCAGTTACTACCT | ARB | 101 | FAM |
| Lactococcaceae | 1252-1267 | 66.3 | ACTGTCTCGCGACTC | ARB | 103 | FAM |
| Helicobacter pylori | 665-682 | 66.1 | CCAAGAATTCCACCTAC | ARB | 103 | FAM |
| Megasphera elsdenii (equivalent to Acidaminococcaceae) | 206-221 | 65.6 | AGCGAAAGCTCCGAA | ARB | 101 | FAM |
| Peptostreptococcaceae (P. productus, equivalent to Ruminococcus productus, P. anaerobicus, P. octavius, P. hereii, P. iviricus, etc.) | 597-615 | 66.4 | TAGCCTTTAACCACCGAC | ARB | 101 | FAM |
| Ruminococcus albus | 82-100 | 66.9 | CTAGCTAGAGAGTGCAAG | ARB | 101 | FAM |
| Ruminococcus gnavus et rel. | 85-99 | 66.2 | CCAAGGCTTCAATC | ARB | 101 | FAM |
| Ruminococcus hansenii et rel. (R. hansenii, R. obeum et al.) | 601-616 | 64.9 | CCAGCCTTTCACATC | ARB | 101 | FAM |
| Staphylococcaceae | 1117-1203 | 66.9 | AGCGCAACCCTTAA | ARB | 103 | FAM |
| Streptococcaceae | 656-671 | 64.8 | CCTTCTGCACTCAA | ARB | 103 | FAM |
Nomenclature according to Bergey's Manual of Determinative Bacteriology (13a).
Escherichia coli reference strain numbering.
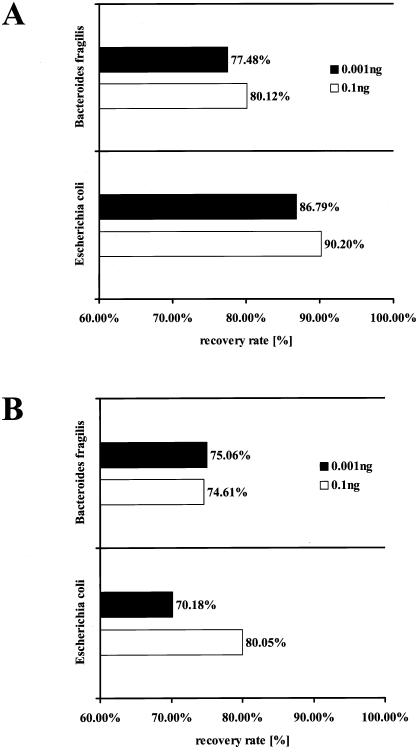
The results of a spiking experiment are shown in Fig. 2. Defined amounts of DNA extracted from Escherichia coli and Bacteroides fragilis (0.1 and 0.001 ng) were spiked into DNA obtained from a biopsy and a stool sample, and the copy number was measured by real-time PCR with the specific probes. The number of cells found in the stool and biopsy samples was subtracted from the number of cells found in the DNA mixture, and the recovery rates were calculated. As indicated in Fig. 2, the mean recovery rate of bacterial DNA was 78.76% (range 70.18 to 90.20%).
FIG. 2.
Recovery rates of different concentrations (0.1 and 0.001 ng) of bacterial DNA (Escherichia coli and Bacteroides fragilis) spiked into DNA of an intestinal biopsy sample (A) and a stool sample (B) of healthy volunteers. The mean recovery of bacterial DNA is 78.76% (range, 70.18 to 90.20%).
Generation of standard curves for quantifying the total number of bacteria and specific bacteria.
For quantification of total bacteria, standard curves for 10 different bacterial strains representing dominant residents of the human gastrointestinal tract were generated with real-time PCR. Total bacteria were quantified with a pair of conserved primers amplifying the full-length 16S rRNA gene and a VIC universal probe (Table 2). Though the bacteria tested had a broad range of rrn copy numbers, the CT values at the different dilution points showed only little variance (13.756 ± 1.05 at 105, 17.792 ± 1.28 at 104, 22.125 ± 1.20 at 103, 26.986 ± 1.10 at 102, 32.047 ± 1.13 at 101, and 37.209 ± 0.14 at 100) suggesting that rrn copy numbers have no major influence on quantification of bacteria. Therefore, only one species (Escherichia coli) was used to generate the standard curve for total bacteria in subsequent experiments (21).
For quantification of specific bacteria, the pair of conserved primers were combined with specific FAM-labeled probes (Table 2). The standard curves for quantification of specific bacteria and bacterial populations were generated with bacterial strains and plasmid 16S rDNA from cloning experiments. The VIC-labeled universal probe was used as the internal positive control for each sample to prove the basic function of the PCR and for relative quantification in connection with a specific probe (Fig. 3).
FIG. 3.
Design of the experimental setup, showing the positions of universal primers and molecular probes. The target sequence is the full-length 16S rDNA. Both fluorescent probes, the VIC-labeled universal probe and the FAM-labeled specific probe, were used in the same reaction with the universal probe operating as an internal positive control (IPC+).
Quantification of clinical samples.
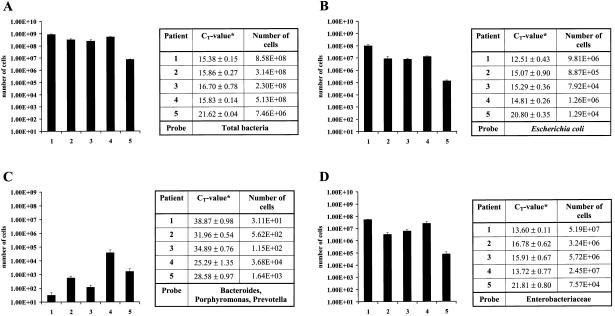
Gut biopsies and stool samples from healthy individuals were used to validate the approach. Specific FAM-labeled probes and the universal VIC-labeled probes were used simultaneously in the same reaction. For the quantification of total bacteria, a range of 1 to 10 ng of DNA is sufficient as a template for each real-time PCR. For the analysis of specific bacteria, 100 to 200 ng of DNA was necessary. Depending on the results, the concentration of DNA was adjusted by further dilution to fall within the linear range of the standard curve. The number of cells was determined according to the standard curve and then normalized to the total number of cells (as determined in the same well with the VIC-labeled probe). Figure 4 shows an example of the distribution of cell quantities found in biopsies of five healthy control patients for total bacteria (A), Escherichia coli (B), Bacteroides, Porphyromonas, and Prevotella (C), and Enterobacteriaceae. The corresponding CT values are also shown.
FIG. 4.
Number of cells detected by real-time PCR in clinical samples (biopsies) of five healthy controls (patients 1 to 5). (A) Total bacteria (VIC-labeled universal probe). (B, C, and D) Escherichia coli; Bacteroides, Porphyromonas, and Prevotella; and Enterobacteriaceae, respectively (FAM-labeled specific probes). Normalized mean values of two independent experiments ± standard deviation are shown.
DISCUSSION
A critical requirement for understanding the intestinal flora is correct and global quantification of its composition. We therefore developed and tested a set of primers, probes, and conditions for TaqMan real-time PCR with the 16S rDNA taxonomic system.
Choice of the experimental system.
Real-time PCR was chosen because this is the most quantitative and reliable tool to determine bacterial concentrations in environmental and clinical samples (5, 10, 13, 15, 21). With real-time PCR, a broad range of quantities from 1 CFU up to 108 CFU can be measured (23). The specificity of detection in the PCR can be provided by specific primers and specific probes. In most previous studies, single pathogens have been detected by real-time PCR with specific primers amplifying a species-specific target sequence (7, 9, 21).
To facilitate global quantification of bacterial species and normalization to a single standard curve of total bacteria in this study, a pair of primers binding to highly conserved regions on the 16S rRNA gene were used to amplify the full-length 16S rDNA. The conserved primers for this real-time PCR were described previously and detect most of the relevant bacteria (35). The number of total bacteria was determined with a VIC-labeled universal probe that was included in all reactions, providing a uniform means of standardization. Specificity is generated exclusively through the specific probe. Amplification of the whole 16S gene is necessary because the regions to identify and define the different bacterial species or groups are distributed over the full length of the 16S rDNA sequence. By using conserved primers, optimization of the PCR was reduced. As the same PCR is used for each reaction, this real-time PCR assay is independent of variations in annealing temperature or composition of the PCR mix due to optimization of the underlying PCR.
Detection and quantification of target DNA sequences by fluorogenic DNA probes usually requires extensive efforts in optimizing reaction conditions. This real-time PCR uses minor groove binder fluorescent probes. DNA probes with conjugated minor groove binder groups form extremely stable duplexes with the target DNA, allowing shorter probes to be used for hybridization-based assays (17). Compared with unmodified DNA probes, MGB probes have higher melting temperature and increased specificity (17). In the present study, nonfluorescent quencher dyes (also called dark quenchers) were used. A nonfluorescent quencher is essentially a chromophore that acts as an energy transfer acceptor from the reporter molecule that does not emit a detectable fluorescent signal of its own, giving a less complicated signal with lower fluorescent background. This improves spectral discrimination and makes data interpretation easier. Due to the increased stability of assays based on MGB technology, optimizing expenditure can be reduced to a minimum. A comparison of MGB probes and probes without a minor groove binder revealed higher sensitivity of the MGB probes (Table 1, Fig. 1). Fluorescent signals of a serial dilution of bacterial DNA from different strains showed that the detection limits of MGB probes for both the VIC-labeled universal probe and FAM-labeled specific probes were 2 log ranks higher than that of probes without a minor groove binder (Fig. 1). Since no experimental data are available in the literature, further experimental work has to be done to support these findings.
Contamination of Taq polymerase with bacterial genomic DNA that is not removed during the purification process is considered a serious problem with use of real-time PCR for bacterial quantification (4, 5, 15, 21, 23). Contamination usually occurs in the last cycles of real-time PCR (after 40 cycles). To circumvent the problem, some authors recommend treating Taq polymerase with DNase I to reduce contamination (21, 23). For this real-time PCR, a ready-to-use PCR master mix (Applied Biosystems) was applied. Negative controls showed no notable contamination with bacterial DNA; nonetheless, CT values of 40 and more were disregarded.
Relevance of rrn operon numbers.
Although the bacteria had different rrn operon copy numbers of the 16S gene, the results at the serial dilution points were very similar, showing only minor variance of CT values. A superimposed standard curve composed of the averaged CT values at the different serial dilution points was generated. For further experiments, only one of the species was used for quantification of total bacteria. An adjustment for rrn operon copy number was not made. There are different concepts to consider the rrn operon numbers in quantitative 16S rDNA-based experimental systems. Lyons et al. found only a small shift in the real-time signal between four different species used for generating a standard curve, suggesting that rrn operon number is a negligible factor in quantification (21). Other authors state that PCR is influenced by variations in the number of rrn operons, which is related to the metabolic status and the generation time of the bacteria at the time of sampling (23, 34). For this real-time PCR, we used bacteria with rrn operon numbers ranging from two (Lactobacillus acidophilus) to 10 to 15 (Clostridium difficile) copy numbers (rrndb, the rRNA-Operon Copy Number Database, http://rrndb.cme.msu.edu) and found only small discrepancies. A correction for artifacts due to the metabolic status of the bacteria and generation time is not realizable in view of the complex metabolic structure of intestinal flora. Hence, we made no attempt to correct the results for rrn copy numbers.
Overall assessment.
DNA from cultured bacteria and cloned 16S rDNA for the 20 species and groups selected was used to calibrate and optimize real-time PCR. Cross-reaction experiments showed no significant overlap between closely related bacterial strains. As specificity is provided only by the probe, spiking experiments were performed to demonstrated the ability of this assay to specifically detect spiked bacterial DNA from a complex genetic background. In PCR amplification of 16S rDNA from complex microbiota, a mixture of homologous molecules serves as the template. Due to different hybridization efficiency and specificity of the primers, amplification efficiencies are not the same for all molecules (34). Thus, the global approach presented here is particularly suitable for relative quantification of bacterial species and populations, because a total bacterial count is measured (universal VIC probe) in each reaction simultaneously with the specific quantification (specific FAM probe) (Fig. 3). However, the necessarily long amplicon also leads to some reduction of sensitivity. Therefore, for bacteria of low abundance, quantification with specific probes and primers may be needed. Validation of the results obtained by real-time PCR with traditional bacterial culture methods is difficult to perform in parallel because of the broad spectrum of specific probes, which detect groups of bacteria rather than single species. The lack of validation may be a shortcoming of the experimental system presented in this study, and thus the global real-time PCR approach should be used for relative quantification rather than absolute quantification.
In summary, the real-time PCR system with universal primers and specific probes presented provides an accurate and stable method to measure bacterial concentrations in clinical samples. A set of 20 specific molecular probes detecting the most frequent bacteria of the human gastrointestinal tract and one universal probe detecting the total number of bacteria were designed and optimized. Determination of both relative and absolute numbers of bacteria is possible. We anticipate that the use of this global quantification tool may facilitate the understanding of the intestinal flora as a whole.
Acknowledgments
This work was supported by grants from the German National Genome Research Network (NGFN), the German Human Genome Project (DHGP), and the Competence Network on Inflammatory Bowel Disease (all funded by the BMBF), the DFG (SFB415), the Crohn′s and Colitis Foundation of America (CCFA), and the European Union (EU QLG2-CT-2001-02161).
We gratefully acknowledge Meike Barche and Gaby Prühs for technical assistance.
REFERENCES
- 1.Amman, R., W. Ludwig, and K.-H. Schleifer. 1995. Phylogentic identification and in situ detection of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biancone, L., I. Monteleone, G. Del Vecchio Blanco, P. Vavassori, and F. Pallone. 2002. Resident bacterial flora and immune system. Dig. Liver Dis. 34:S37-43. [DOI] [PubMed] [Google Scholar]
- 3.Bjorksten, B., E. Sepp, K. Julge, T. Voor, and M. Mikelsaar. 2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108:516-520. [DOI] [PubMed] [Google Scholar]
- 4.Corless, C. E., M. Guiver, R. Borrow, V. Edwards-Jones, E. B. Kaczmarski, and A. J. Fox. 2000. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J. Clin. Microbiol. 38:1747-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang, Y., W. H. Wu, J. L. Pepper, J. L. Larsen, S. A. Marras, E. A. Nelson, W. B. Epperson, and J. Christopher-Hennings. 2002. Comparison of real-time, quantitative PCR with molecular beacons to nested PCR and culture methods for detection of Mycobacterium avium subsp. paratuberculosis in bovine fecal samples. J. Clin. Microbiol. 40:287-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell, R. J., and J. T. LaMont. 2002. Microbial factors in inflammatory bowel disease. Gastroenterol. Clin. North Am. 31:41-62. [DOI] [PubMed] [Google Scholar]
- 7.Fortin, N. Y., A. Mulchandani, and W. Chen. 2001. Use of real-time polymerase chain reaction and molecular beacons for the detection of Escherichia coli O157:H7. Anal. Biochem. 289:281-288. [DOI] [PubMed] [Google Scholar]
- 8.Göbel, U. B. 1995. Phylogenetic amplification for the detection of uncultured bacteria and the analysis of complex microbiota. J. Microbiol. Methods 23:117-128. [Google Scholar]
- 9.Greiner, O., P. J. R. Day, P. P. Bosshard, F. Imeri, M. Altwegg, and D. Nadal. 2001. Quantitative detection of Streptococcus pneumoniae in nasopharyngeal secretions by real-time PCR. J. Clin. Microbiol. 39:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guiver, M., R. Borrow, J. Marsh, S. J. Gray, E. B. Kaczmarski, D. Howells, P. Boseley, and A. J. Fox. 2000. Evaluation of the Applied Biosystems automated Taqman polymerase chain reaction system for the detection of meningococcal DNA. FEMS Immunol. Med. Microbiol. 28:173-179. [DOI] [PubMed] [Google Scholar]
- 11.Hampe, J., A. Cuthbert, P. J. Croucher, M. M. Mirza, S. Mascheretti, S. Fisher, H. Frenzel, K. King, A. Hasselmeyer, A. J. MacPherson, S. Bridger, S. van Deventer, A. Forbes, S. Nikolaus, J. E. Lennard-Jones, U. R. Foelsch, M. Krawczak, C. Lewis, S. Schreiber, and C. G. Mathew. 2001. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357:1925-1928. [DOI] [PubMed] [Google Scholar]
- 12.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, J. A., Z. Hubalek, J. Halouzka, K. L. Elkins, A. Sjostedt, M. Shipley, and M. S. Ibrahim. 2000. Detection of Francisella tularensis in infected mammals and vectors using a probe-based polymerase chain reaction. Am. J. Trop. Med. Hyg. 62:310-318. [DOI] [PubMed] [Google Scholar]
- 13a.Holt, J. G. (ed.). 1994. Bergey’s manual of determinative bacteriology. Williams & Wilkins, Baltimore, Md.
- 14.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 15.Huijsdens, X. W., R. K. Linskens, M. Mak, S. G. Meuwissen, C. M. Vandenbroucke-Grauls, and P. H. Savelkoul. 2002. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. J. Clin. Microbiol. 40:4423-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalliomaki, M., and E. Isolauri. 2002. Pandemic of atopic diseases—a lack of microbial exposure in early infancy? Curr. Drug Targets Infect. Disord. 2:193-199. [DOI] [PubMed] [Google Scholar]
- 17.Kutyavin, I. V., I. A. Afonina, A. Mills, V. V. Gorn, E. A. Lukhtanov, E. S. Belousov, M. J. Singer, D. K. Walburger, S. G. Lokhov, A. A. Gall, R. Dempcy, M. W. Reed, R. B. Meyer, and J. Hedgpeth. 2000. 3′-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res. 28:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langendijk, P., F. Schut, G. Jansen, G. Raangs, G. Kamphuis, M. Wilkinson, and G. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linskens, R. K., X. W. Huijsdens, P. H. Savelkoul, C. M. Vandenbroucke-Grauls, and S. G. Meuwissen. 2001. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand. J. Gastroenterol. Suppl. 234:29-40. [DOI] [PubMed]
- 20.Ludwig, W., O. Strunk, S. Klugbauer, N. Klugbauer, M. Weizenegger, J. Neumaier, M. Bachleitner, and K. H. Schleifer. 1998. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19:554-568. [DOI] [PubMed] [Google Scholar]
- 21.Lyons, S. R., A. L. Griffen, and E. J. Leys. 2000. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J. Clin. Microbiol. 38:2362-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarlene, G., and G. Gibson. 1994. Metabolic activities of the normal colonic microflora, p. 17-38. In S. Gibson (ed.), Human health: contribution of microorganisms. Springer, Frankfurt, Germany.
- 23.Nadkarni, M. A., F. E. Martin, N. A. Jacques, and N. Hunter. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257-266. [DOI] [PubMed] [Google Scholar]
- 24.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 25.Sartor, R. B. 1997. Enteric microflora in IBD: pathogens or commensals? Inflamm. Bowel Dis. 3:230-235. [PubMed] [Google Scholar]
- 26.Sartor, R. B. 1997. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res. Immunol. 148:567-576. [DOI] [PubMed] [Google Scholar]
- 27.Sen, K. 2000. Rapid identification of Yersinia enterocolitica in blood by the 5′ nuclease PCR assay. J. Clin. Microbiol. 38:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen, S., M. M. Mullan, T. J. Parker, J. T. Woolner, S. A. Tarry, and J. O. Hunter. 2002. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig. Dis. Sci. 47:2615-2620. [DOI] [PubMed] [Google Scholar]
- 29.Simon, G. L., and S. L. Gorbach. 1984. Intestinal flora in health and disease. Gastroenterology 86:174-193. [PubMed] [Google Scholar]
- 30.Tannock, G. W., A. Tilsala-Timisjarvi, S. Rodtong, J. Ng, K. Munro, and T. Alatossava. 1999. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl. Environ. Microbiol. 65:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson, W. G. 2001. Probiotics for irritable bowel syndrome: a light in the darkness? Eur. J. Gastroenterol. Hepatol. 13:1135-1136. [DOI] [PubMed] [Google Scholar]
- 32.Uhari, M. 2002. Review: Lactobacillus is safe and effective for treating children with acute infectious diarrhea. ACP J. Club 137:96.. [PubMed] [Google Scholar]
- 33.Van Niel, C. W., C. Feudtner, M. M. Garrison, and D. A. Christakis. 2002. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 109:678-684. [DOI] [PubMed] [Google Scholar]
- 34.von Wintzingerode, F., U. B. Gobel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 35.von Wintzingerode, F., O. Landt, A. Ehrlich, and U. B. Gobel. 2000. Peptide nucleic acid-mediated PCR clamping as a useful supplement in the determination of microbial diversity. Appl. Environ. Microbiol. 66:549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson, K., and R. Blitchington. 1996. Human colonic biota studied by ribosomal DNA sequence analysis. Appl. Environ. Microbiol. 62:2273-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woese, C. R., and G. E. Fox. 1977. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. USA 74:5088-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]