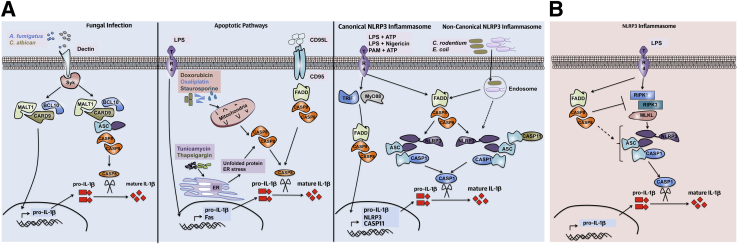
Figure 2.
Novel roles for caspase-8 in positive (A) and negative (B) regulation of IL-1β and the NLRP3 inflammasome. A: Fungal infection (left panel) induces signaling through the dectin receptor and Syk activation. CARD9–BCL10–MALT1 complex formation induces NF-κB signaling and up-regulates pro–IL-1β. Recruitment of apoptosis-associated speck-like protein containing CARD (ASC) and caspase-8 to the CARD9–BCL10–MALT1 complex induces caspase-8 activation, which then cleaves pro–IL-1β to its mature form. Induction of apoptosis (middle panel) in the presence of LPS priming is accompanied by caspase-8–dependent IL-1β processing. CD95L signals through CD95 to recruit FADD and caspase-8 to the receptor and to induce apoptosis. In the presence of LPS, CD95L induces activation of caspase-8 and caspase-8–dependent IL-1β release. Chemotherapeutic drugs induce mitochondrial dependent intrinsic cell death. In the presence of LPS, doxorubicin and oxaliplatin induce caspase-8 activation. Treatment of cells with tunicamycin and thapsigargin [known inducers of endoplasmic reticulum (ER) stress] in the presence of LPS triggers caspase-8 activation. Active caspase-8 cleaves pro–IL-1β to release mature IL-1β. In the NLRP3 inflammasome (right panel), caspase-8 is required for both canonical and noncanonical NLRP3 inflammasome activation. Stimulation of TLR results in NF-κB activation and up-regulation of pro–IL-1β and NLRP3 mRNA that is partially dependent on caspase-8. Stimulation of the canonical NLRP3 (LPS+ATP, LPS+nigericin, Pam3CSK4+ATP) or noncanonical NLRP3 (C. rodentium, E. coli) inflammasome requires caspase-8 for assembly and activation of the NLRP3 inflammasome complex. Caspase-8 activation is required for activation of caspase-1 and caspase-11. B: Caspase-8 is a negative regulator of LPS-induced activation of the NLRP3 inflammasome. In the absence of caspase-8, dendritic cells are hyper-responsive to LPS stimulation and activate the NLRP3 inflammasome in a RIPK1-, RIPK3-, and MLKL-dependent manner. PAM, Pam3CSK4.