Abstract
Fibrosis, with resultant loss of organ function, is the endpoint of many diseases. Despite this, no effective anti-fibrotic therapies exist. The myofibroblast is the key cell driving fibrosis but its origins remain controversial. A growing body of work provides strong evidence that the pericyte, a perivascular cell present throughout the microvasculature, is a major myofibroblast precursor in multiple tissues. This review summarizes the principle experimental and clinical evidence underpinning this conclusion and outlines strategies for targeting pericyte transdifferentiation during fibrogenesis. Successful targeting of pro-fibrogenic pericytes has the potential to halt or even reverse fibrosis and thus reduce the enormous worldwide healthcare burden that currently exists as a result of fibrotic disease.
Introduction
Fibrosis is a major healthcare burden worldwide. As a highly conserved, stereotyped response to chronic, repetitive injury, characterized by excessive accumulation of extracellular matrix, fibrosis represents the end-stage of a plethora of disease processes across multiple organ systems. Diseases as diverse as chronic kidney disease, viral and metabolic liver disease, systemic sclerosis, pulmonary fibrosis, myocardial infarction, stroke and cancer all have a major fibrotic component to their pathology. It is thus all the more remarkable that there are currently no effective therapies available to reverse or even halt the insidious progression of fibrosis and, ultimately, irreversible loss of organ function.
Key to progressing our knowledge of fibrosis is an improved understanding of the central cell type driving fibrogenesis—the myofibroblast. Most pertinently, identifying the cell type(s) from which the myofibroblast originates should open a range of therapeutic avenues targeting myofibroblast generation and the development of fibrosis. Recently, the pericyte has gained increasing attention as a major myofibroblast progenitor and this review summarizes recent experimental and clinical research in multiple organ systems highlighting the central role of pericytes in the pathogenesis of tissue fibrosis.
Origins of fibrosis
While the myofibroblast is generally accepted as the central mediator of fibrosis due to its principle functions of extracellular matrix deposition and exertion of tensile force, its origins remain controversial.1 A variety of cells and processes have been proposed as the principle source of myofibroblasts, including tissue-resident cells, bone marrow-derived cells, epithelial cells undergoing epithelial-mesenchymal transition (EMT) and endothelial cells undergoing endothelial-mesenchymal transition (EndMT).2–5 EMT had been proposed as the major source of myofibroblasts, but a series of fate-mapping studies in rodent models of fibrosis in a range of tissues has provided strong evidence that EMT does not contribute to the myofibroblast population during fibrogenesis in vivo.6,7 Conversely, several studies using cutting-edge genetic cell-labelling techniques have provided evidence that the pericyte is a major myofibroblast progenitor in a range of organs.7–10
What are pericytes?
The pericyte is a perivascular cell, ubiquitously located on the abluminal surface of the microvasculature (capillaries, precapillary arterioles and postcapillary venules) and embedded (at least in part) in the microvascular basement membrane.11 Multiple, long cytoplasmic processes permit contact with multiple endothelial cells and relate to their primary role in vascular stabilization and angiogenesis. In health, pericytes can be definitively identified anatomically using electron microscopy, although this is of limited utility in the standard research setting. The changes that occur in the context of fibrosis, with basement membrane turnover and migration of the pericyte away from its perivascular location, increase the challenges of accurate identification, as does the lack of a single marker entirely specific for, and consistently expressed by, all pericytes. Commonly utilized markers include platelet-derived growth factor receptor beta (PDGFRβ), nerve/glial antigen 2 (NG2), desmin and alpha-smooth muscle actin (αSMA),11 although it should also be noted that αSMA is a classical myofibroblast marker. The difficulties of identifying and subsequently tracking pericytes throughout the development of fibrosis has hampered research using human tissues and led researchers to utilize transgenic, rodent based, cell-labelling techniques to permit fate-mapping of pericytes and thus, through mechanistic studies, advance our knowledge of the pericyte’s role in fibrosis.12
Renal pericytes
A substantial proportion of the existing research into the role of pericytes in fibrosis has been undertaken in the kidney, albeit predominantly in animal models. Lin et al.13 labelled collagen-α1(I)-expressing cells in the murine kidney through their concomitant expression of green fluorescent protein (GFP). Co-staining with the pericyte markers NG2, PDGFRβ and αSMA allowed identification of pericytes and perivascular fibroblasts in the uninjured kidney and provided evidence that these cells contribute to the population of interstitial myofibroblasts induced by experimental renal fibrosis. Subsequently, Humphreys et al.7 published a true fate-mapping study in which renal stromal cells (comprising mesangial cells, vascular smooth muscle cells and pericytes) in the mouse were heritably labelled by targeting their FoxD1 expression with a Cre recombinase reporter strategy, such that only this cell population expressed the LacZ reporter gene. In the interstitium of the uninjured adult kidney these labelled cells co-expressed the pericyte/perivascular fibroblast markers PDGFRβ and CD73. Following induction of renal fibrosis through unilateral ureteric obstruction, the number of labelled cells increased markedly and comprised almost all the population of αSMA+ myofibroblasts. The study also heritably labelled renal epithelial cells and found no evidence that the epithelial cell population contributed to the myofibroblast population through EMT.
In human kidney, the role of pericytes in chronic kidney disease has been explored using the stromal marker CD248 (Endosialin or TEM1).14 In healthy kidney (human and murine), CD248 expression was observed in pericytes and stromal fibroblasts. In 93 patients with IgA nephropathy, diffuse tubulointerstitial staining for CD248 was observed and expression correlated with disease severity (as assessed by urinary albumin-creatinine ratio, degree of fibrosis and estimated glomerular filtration rate) and was shown to be an independent predictor of renal survival. A subpopulation of αSMA+ myofibroblasts expressed CD248 with αSMA+ CD248– myofibroblasts and αSMA– CD248+ fibroblasts also observed. Thus this study provides observational evidence that at least part of the myofibroblast population arising during interstitial renal fibrosis in humans may derive from a pericyte population. The link between CD248 expression and disease severity and progression certainly merits further investigation.
Liver-specific pericytes
Hepatic stellate cells (HSCs) are liver-specific pericytes that reside within the space of Disse, in close contact with sinusoidal endothelial cells. A central role for these cells in fibrosis was discovered almost three decades ago, when isolated HSCs were shown to be major collagen-producing cells of the liver.15 Kisseleva et al.16 provided further evidence that HSCs are the major source of myofibroblasts in a murine model of liver fibrosis by fluorescently labelling collagen-producing cells with GFP and subsequently showing that 92% of these cells co-expressed vitamin A (detected through autofluoresence), a hallmark of HSCs. Most recently, Mederacke et al.10 employed a novel genetic labelling strategy, again making use of the retinol pathway, to limit fluorescent reporter expression to those cells expressing the Lrat (lecithin-retinol acyltransferase) gene. Using this approach HSCs were labelled heritably and with high specificity. In multiple models of liver injury (toxic, cholestatic and fatty liver disease), the authors confirmed in vivo that HSCs are the principle contributors to the myofibroblast pool. Co-staining with αSMA as a myofibroblast marker and using mice that expressed a fluorescent reporter in collagen-producing cells, they demonstrated that HSCs gave rise to 82–96% of myofibroblasts.
Given the central role of HSCs in liver fibrosis, it is logical to postulate that targeting these cells should have an anti-fibrotic effect. Recently, Henderson et al.17 targeted HSC αv integrins and demonstrated that both genetic ablation of HSC αv expression and pharmacological blockade of αv integrins with a small molecule inhibitor had an anti-fibrotic effect (both protective and ameliorative). This effect was, at least in part, secondary to a reduction in αv integrin-mediated activation of the pro-fibrogenic cytokine transforming growth factor beta (TGFβ). Further, this approach also had an anti-fibrotic effect in models of renal and pulmonary fibrosis, identifying a core, targetable molecular pathway that regulates fibrosis across solid organs.
Pericytes in the skin
Systemic sclerosis (SSc) is an autoimmune condition characterized by vascular injury, inflammation and most importantly fibrosis, not only in the skin but throughout the body, notably the heart, lung and kidney. Although the skin is an inherently accessible organ for study, determining the cellular origins of myofibroblasts in human patients is again limited by the inability to trace the lineage of these activated and differentiated cells. Instead, studies have relied on shared marker expression and histological description to provide observational evidence of myofibroblast origins. Analysis of biopsy samples obtained from patients with excessive dermal scarring was performed by Sundberg et al.,18 with co-staining for pericyte and myofibroblast markers, including PDGFRβ, αSMA and a marker of collagen synthesis, prolyl-4-hydroxylase beta-subunit (P-4-H). This provided evidence suggesting that a population of pericytes might migrate from the perivascular location and begin to synthesize collagen. Furthermore, increased expression of the pericyte marker RGS5 (regulator of G-protein signalling 5) has been shown in scleroderma patients, both perivascularly and within the dermal matrix in cells thought to be myofibroblasts.19 This is supported by the finding that, in the bleomycin-induced mouse model of SSc, the majority of αSMA+ myofibroblasts express the pericyte marker NG2.20
However, the postulated role for pericytes as myofibroblast precursors in dermal fibrosis has recently been substantially reinforced by the elegant work of Dulauroy et al.9 in which the progeny of ADAM12+ (a disintegrin and metalloprotease 12) cells were fluorescently labelled using an inducible Cre system. ADAM12 is a metalloprotease whose expression is restricted to embryogenesis and fibrotic disease. These studies demonstrated that the majority of collagen-producing, αSMA+ myofibroblasts developing following acute dermal or muscle injury are generated from tissue-resident ADAM12+ cells and furthermore from a PDGFRβ+ NG2+ perivascular subpopulation. Thus, this work provides strong evidence that pericytes are a major contributor to the myofibroblast population that develops during skin and muscle scarring.
Therapeutic targeting of pericytes in fibrosis
It is clear that pericytes have a key role as myofibroblast progenitors across a number of organs and disease states. The next major challenge is the translation of this knowledge into potent anti-fibrotic therapies for human disease. The potential for benefit is great, since targeting the pericyte is likely to be of broad clinical utility in the treatment of a range of fibrotic diseases across multiple organs. Furthermore, intervening at the critical stage in pericyte migration and transdifferentiation has the potential to result in synergistic benefits in addition to anti-fibrotic effects. For example, a reduction in the capillary rarefaction that accompanies pericyte migration, a prominent feature of renal fibrosis and scleroderma, and the promotion of tissue regeneration are both realistic aims. However, it will be crucial to target interventions to ensure that effects on the pericyte’s principle and vital homeostatic functions in relation to vascular integrity are negligible.
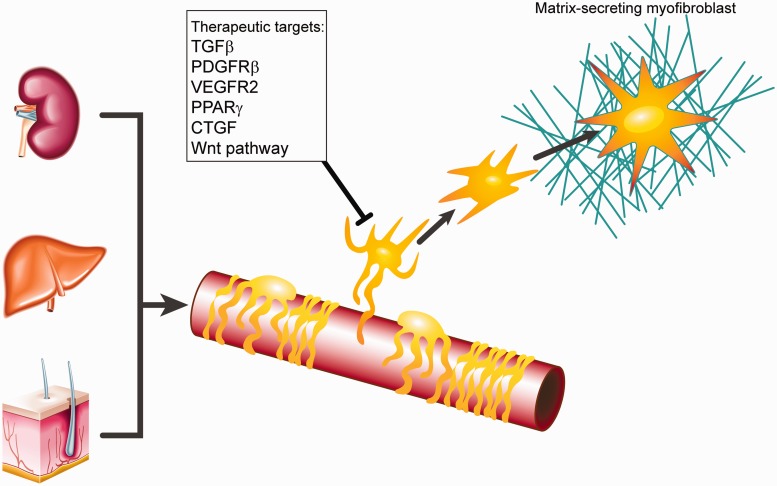
Several possible routes to targeting pericytes are currently being evaluated (Figure 1).21–23 The TGFβ signalling pathway is of particular interest, although targeting of this pathway should be tightly controlled, both spatially and temporally, given the potential for detrimental autoimmune and oncogenic effects from pan-TGFβ blockade. Limiting activation of latent TGFβ by targeting the αv family of integrins is one such example of a more refined approach that has recently shown promise.17 Blocking PDGFRβ or vascular endothelial growth factor receptor-2 (VEGFR2) signalling pathways has also shown potential to attenuate renal fibrosis and capillary rarefaction.24 Indeed, the PDGFRβ inhibitor imatinib has been trialled in systemic scleroderma, albeit with an incidence of adverse events which may limit its clinical utility.21 The Wnt pathway is also garnering increasing interest, with inhibitors of both downstream elements and key co-receptors shown to reduce fibrosis in renal and pulmonary models and inhibit pericyte activation, migration and transdifferentiation to a myofibroblast phenotype.23
Figure 1.
Following injury pericytes in multiple organs, including kidney, liver and skin, can migrate from their usual perivascular location and transdifferentiate into matrix-secreting myofibroblasts. Potential therapeutic targets to inhibit this process are shown. TGFβ, transforming growth factor beta; PDGFRβ, platelet-derived growth factor receptor beta; VEGFR2, vascular endothelial growth factor receptor 2; PPARγ, peroxisome proliferator-activated receptor gamma; CTGF, connective tissue growth factor.
Pericytes also produce connective tissue growth factor (CTGF, CCN2) following injury and blockade of CTGF has been shown to ameliorate skin and lung fibrosis in murine models.22 Clinical trials of anti-CTGF antibody for liver fibrosis and pulmonary fibrosis are in progress, although a phase II trial in diabetic nephropathy has been terminated.23 In liver, the possibility of stimulating the peroxisome proliferator-activated receptor-γ (PPARγ) with agents such as thiazolidinediones in order to modulate HSC activation has recently been reviewed in detail.25
These are exciting times for the fields of pericyte biology and fibrosis, with an increasing body of evidence implicating these perivascular cells as a major source of myofibroblasts in a range of fibrotic diseases across multiple tissues. Common basic pathways leading to pericyte transdifferentiation appear to be conserved across organs and thus a single therapeutic strategy may have broad utility in a number of fibrotic diseases. A greater challenge lies in the discovery of markers that permit definitive identification of pericytes in different contexts, including differentiation from resident tissue fibroblasts. It will also be important to characterize further the pericyte subpopulations present during fibrogenesis. This will help elucidate the mechanisms underlying acquisition of the fibrogenic phenotype, allowing development of highly targeted therapies directed specifically at the pro-fibrogenic pericyte subpopulation. Precise and accurate phenotyping of pericytes, both in health and fibrosis, should ultimately pave the way for successful translation of this rapidly evolving field into potent anti-fibrotic therapies.
Acknowledgements
The authors thank Dr Sree Thayalasekaran for helpful discussions during the preparation of this review.
Funding
This work was supported by the Wellcome Trust and Medical Research Council (UK).
Conflict of interest: None declared.
References
- 1.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–50. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–7. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–36. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 9.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–70. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 10.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh SN, Iredale JP, Henderson NC. Origins of fibrosis: pericytes take centre stage. F1000Prime Rep. 2013;5:37. doi: 10.12703/P5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–27. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, et al. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney Int. 2011;80:199–207. doi: 10.1038/ki.2011.103. [DOI] [PubMed] [Google Scholar]
- 15.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681–5. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA. 2012;109:9448–53. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundberg C, Ivarsson M, Gerdin B, Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest. 1996;74:452–66. [PubMed] [Google Scholar]
- 19.Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One. 2008;3:e1452. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Taghavi R, Leask A. Connective tissue growth factor is induced in bleomycin-induced skin scleroderma. J Cell Commun Signal. 2010;4:25–30. doi: 10.1007/s12079-009-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leask A. Getting out of a sticky situation: targeting the myofibroblast in scleroderma. Open Rheumatol J. 2012;6:163–9. doi: 10.2174/1874312901206010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol. 2012;32:463–70. doi: 10.1016/j.semnephrol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramann R, Dirocco DP, Maarouf OH, Humphreys BD. Matrix producing cells in chronic kidney disease: origin, regulation, and activation. Curr Pathobiol Rep. 2013;1:301–11. doi: 10.1007/s40139-013-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–23. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang F, Kong D, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-γ as a therapeutic target for hepatic fibrosis: from bench to bedside. Cell Mol Life Sci. 2013;70:259–76. doi: 10.1007/s00018-012-1046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]