Figure 2. The effect of PEP005 in two human xenograft mouse models of AML.
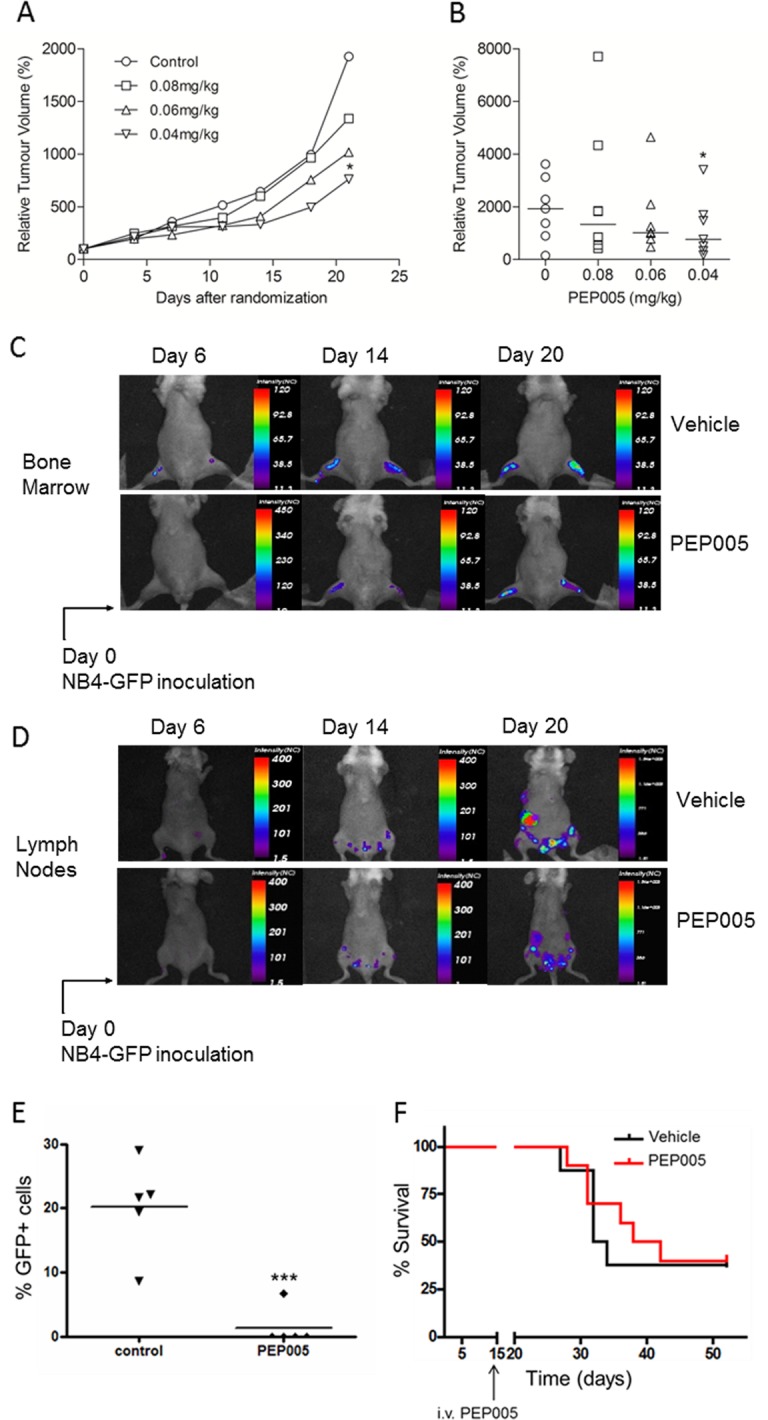
U937 cells were injected subcutaneously into nude mice (A and B). Once cells had formed palpable tumours mice were randomized into four groups (8 mice per group) and different doses of PEP005 treatment were initiated. PEP005 was administered to mice by intravenous injection 3 times a week for 2 weeks. A. Tumour volumes were measured twice a week from day 1, and relative tumour volumes were calculated by (tumour size at day x/tumour size at day 0) × 100. Each point represents the median value of relative tumour volumes. B. Data are shown for individual animals at day 21. The horizontal line represents the median value. * indicates p<0.05. (C-F) GFP expressing NB4 cells were intravenously transplanted in to NOD/SCID/β2mnull mice. After 2 weeks inoculation, mice were divided into two groups (6 mice per group). Vehicle control or 50 mg/kg of PEP005 was administered to mice by intravenous injection daily for 5 days. Recipient mice were monitored and sacrificed when moribund, as defined by weight loss, lethargy and/or paralysis. Images showing GFP-NB4 cell engraftment in the bone marrow (C) and lymph nodes (D) following vehicle or PEP005 treatment. Prior to imaging, anaesthetised mice were depilated and moved to the heated translational stage of the eXpore Optix™. Animals were subsequently maintained under gas anaesthesia during scanning. GFP fluorescence images were generated using Optiview™ software. E. Inguinal lymph nodes were collected after mice were sacrificed and GFP positive (GFP+) cells were enumerated by flow cytometry. Data were analyzed by Flowjo software. *** indicates p<0.001 using two-way ANOVA. F. Survival curves in response to either vehicle (black) or PEP005 (red) treatment.
