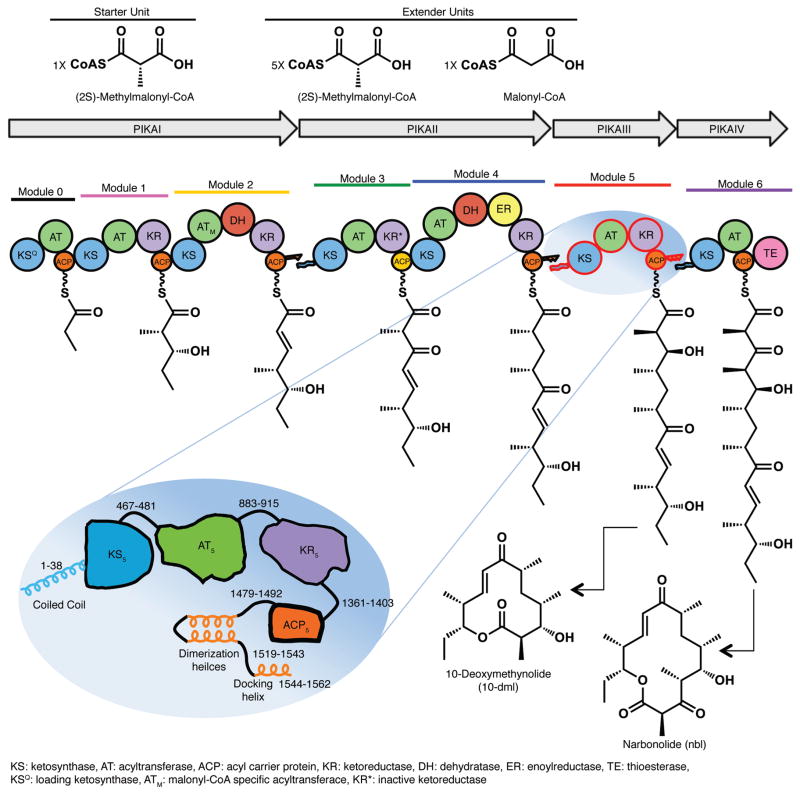
Figure 1.
Modular polyketide synthase for pikromycin. The six modules of the pikromycin PKS, comprised of PikAI-IV polypeptides, sequentially elongate and modify a polyketide intermediate. A polyketide product, either 10-deoxymethynolide (10-dml) from module 5 or narbonolide (nbl) from module 6, is off-loaded by the thioesterase domain (TE) of the final module, PikAIV. Modules are differently colored. Circles represent protein domains (ketosynthase KS, acyltransferase AT, dehydratase DH, enoyl reductase ER, ketoreductase KR and acyl carrier protein ACP; KSQ is a decarboxylase; KR* is inactive), and docking domains are shown as jagged ends. PikAIII schematic: The 1562-amino acid PikAIII polypeptide, selected for this study, is shown with functional domains in contrasting colors, used throughout, and linker peptides identified by residue ranges. The N- and C-terminal docking domains are shown as helices, as are the post-ACP dimerization helices.