Figure 3.
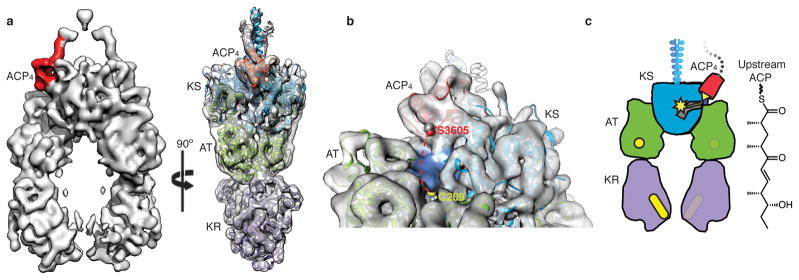
Interaction of upstream ACP with the PKS module. a, Orthogonal views of solid rendering (left) and transparent representation with modeled structures (right) of the cryo-EM map of pentaketide-ACP4-PikAIII/C209A/ΔACP5 at 8.6 Å resolution. b, The position of Ser3605 proximal to the KS active site entrance. Ser3605 (red sphere) and Cys209 (yellow sphere) are 28 Å apart (dashed red line). Loops 1 and 2 of ACP4 (residues 3588-3606 and 3624-3634) contact two helices (residues 284-293 and 316-322) and a loop (residues 140-150) of KS5. c, Cartoon representation of pentaketide-ACP4-PikAIII/C209A/ΔACP5. The upstream ACP (red with yellow serine) carrying the pentaketide intermediate (yellow line) docks to the side entrance of the downstream KS (blue with yellow active site).
