Figure 4.
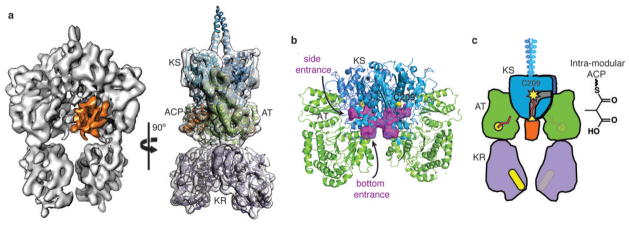
Interaction of intra-module ACP5 with KS5 in methylmalonyl-PikAIII. a, Orthogonal views of solid rendering (left) and transparent representation with modeled structures (right) of the MM-PikAIII cryo-EM map at 7.3-Å resolution. The ACP (orange) has shifted ~20 Å relative to its position in holo-PikAIII conformer II. b, KS active site channels. Internal cavity analysis (purple surface) depicts channels to the active site from both the side and bottom entrances. c, Cartoon representation of MM-PikAIII. AT (green, yellow active site) loading of the MM building block (red) onto the intra-module ACP (orange, yellow serine) positions the carrier domain at the bottom entrance of KS (blue, yellow active site) for decarboxylative condensation, remote from the KR domain (purple, yellow active site).
