Abstract Abstract
Venous thromboembolism (VTE) encompasses deep-vein thrombosis and pulmonary embolism (PE). It is the third-most-frequent cardiovascular disease, with an overall annual incidence of 1–2 per 1,000 population. Chronic thromboembolic pulmonary hypertension (CTEPH) is regarded as a late sequela of PE, with a reported incidence varying between 0.1% and 9.1% of those surviving acute VTE. Right ventricular (RV) function is dependent on afterload. The most precise technique to describe RV function is invasive assessment of the RV–to–pulmonary vascular coupling. However, assessments of RV afterload (i.e., steady and pulsatile flow components and their product, the RC-time) may be useful hemodynamic surrogates of coupling. RV load is different in acute and chronic PE. In acute PE, more than 60% occlusion of the cross-sectional area of the pulmonary artery within a short period of time leads to abrupt hemodynamic collapse. If the time of occlusion is limited to ∼15 seconds, significant decreases in fractional area change, tricuspid annulus systolic excursion, and RV free-wall deformation (strain) occur, with the latter showing significant postsystolic shortening. These changes have similarities to ischemic stunning, and they recover within minutes. In CTEPH, studies of pulmonary vascular resistance (PVR) and pulmonary arterial compliance demonstrated low RC-times that were further lowered after pulmonary endarterectomy (PEA). Immediate postoperative PVR was the only predictor of long-term survival/freedom from lung transplantation, suggesting that the effect of PEA on opening vascular territories to flow outweighs its effect on proximal stiffness. This review summarizes the current knowledge on vascular and intrinsic RV adaptation to VTE, including CTEPH, and the role of imaging.
Keywords: right ventricle, hemodynamics, pulmonary heart disease, pulmonary embolism
Venous thromboembolism (VTE) encompasses deep-vein thrombosis (DVT) and pulmonary embolism (PE). It is the third-most-frequent cardiovascular disease, with an overall annual incidence of 1–2 per 1,000 population. Chronic thromboembolic pulmonary hypertension (CTEPH) is regarded as a late sequela of PE. Right ventricular (RV) function is an important determinant of long-term outcome in patients with acute PE and CTEPH. In these conditions, the right ventricle (RV) is subjected to abnormal and increased loading that varies in timing, magnitude, and duration. Consequently, RV dysfunction and pulmonary hypertension (PH) are variably present at initial presentation of acute PE. After an episode of PE, pulmonary hemodynamics and RV function normalize within a few weeks in the majority of patients. CTEPH results from persistence of thrombotic obstructions in the pulmonary vasculature in the presence of significant positive and obliterative vascular remodeling and chronic elevation in pulmonary pressures. CTEPH leads to a progressive increase in RV afterload, causing RV dysfunction and eventually RV failure and death. The condition is underdiagnosed, and the true prevalence is still unknown. Prognosis of acute PE and development of CTEPH can be predicted by pulmonary artery pressures and RV function at the time of diagnosis of the first episode of PE.1 While noninvasive diagnostics are performed in acute PE, right heart catheterization and pulmonary angiography remain gold standards for the diagnosis of CTEPH.
Invasive assessment of RV afterload
RV function is dependent on RV afterload, which consists of pulmonary vascular resistance (PVR; steady flow load of the RV), pulmonary arterial compliance (CPA; oscillatory load of the RV during systole), and characteristic impedance (Z) of the proximal pulmonary artery.2 RV hydraulic load is determined by the dynamic interaction between PVR and CPA.3 RC-time, the product of resistance and compliance, represents the exponential pressure decay in the pulmonary artery during diastole.4 The CPA relates to oscillatory load and has been shown to be of greater prognostic importance than resistance5 and to be associated with RV dysfunction5,6 in patients with idiopathic pulmonary arterial hypertension (iPAH).
By means of the pulmonary artery occlusion technique, the decay from pulmonary artery pressure level to pulmonary arterial wedge pressure level can be assessed to estimate the pressure in precapillary small pulmonary arteries (POCCL).7-9 With POCCL, PVR can be partitioned into larger-arterial (upstream resistance) and small-arterial plus venous (downstream resistance) components7-9 with the pulmonary artery occlusion technique.
Invasive assessment of RV function
RV function can be directly measured by conductance catheterization and an instantaneous integration of RV pressure, and pulmonary arterial flow (so-called pressure-volume loops) can be derived. Diastolic RV function can be assessed by RV end-diastolic pressure and volume, the minimal rate of RV pressure change (dP/dtmin), the RV stiffness constant (β), and the isovolumetric relaxation time constant (τ).10-12 The adaptation of the RV to its afterload can be assessed by quantification of the coupling of RV systolic function to arterial elastance. Two measures are critical for the assessment of right ventricular–to–pulmonary vascular (RV-PV) coupling: (1) end-systolic ventricular elastance (Ees = end-systolic pressure/end-systolic volume), which is the best possible load-independent measure of contractility, and (2) arterial elastance (Ea = end-systolic pressure/stroke volume [SV]), a measure of afterload that opposes the RV. The Ees and Ea can be graphically derived from pressure-volume loops of the RV.13 RV-PV coupling can be calculated by dividing Ees by Ea (Ees/Ea ratio). The optimal matching of systolic ventricular and arterial elastances occurs at an Ees/Ea ratio of ∼1.5. An isolated increase in Ea or decrease in Ees leads to a decrease in Ees/Ea ratio, indicating decoupling of the ventricle from its arterial system and a decrease in SV. However, in contrast to that in the left ventricle (LV), measurement of Ees in the RV might be inaccurate because of the complex geometry of the RV and the triangular shape of the RV pressure-volume loop, resulting from the high compliance of the pulmonary vasculature14 and from the fact that RV ejection continues after end-systole. This limitation can be resolved by measuring pressure-volume loops during preload reduction of the RV by temporary balloon occlusion of the inferior vena cava. However, this method is very invasive and may cause alterations in sympathetic tone, thus leading to alterations in hemodynamics. An alternative method that has been proposed to assess Ees without the need for preload reduction is the single-beat method.15,16 With the single-beat method, the maximal ventricular pressure (Pmax), as encountered in an isovolumetric nonejecting beat, can be estimated by fitting a sinus wave over the RV pressure curve during the isovolumetric contraction-and-relaxation phase (Fig. 1). The Ees can be derived from Pmax by dividing the difference between Pmax and mean pulmonary artery pressure (mPAP) by SV.12,17 The Ea can be estimated as mPAP/SV.12,17 Brimioulle et al.16 found an excellent correlation between Pmax predicted by the single-beat method and Pmax derived from pressure-volume loops during preload reduction in dogs. Animal studies have shown that RV-PV coupling is preserved in survivors of acute PE,18 while it is decoupled in models of chronic pressure overload due to recurrent embolization.19 Reduced ventriculoarterial coupling efficiency has been shown in recent clinical studies in different forms of PH.20-22 Intrinsic RV dysfunction contributing to more severe decoupling could be demonstrated in patients with scleroderma-associated pulmonary arterial hypertension (PAH), compared to patients with iPAH.22 Recent data suggest that the RV is decoupled from the pulmonary vasculature in patients with CTEPH and in those with chronic thromboembolic pulmonary vascular disease (CTPVD).23
Figure 1.
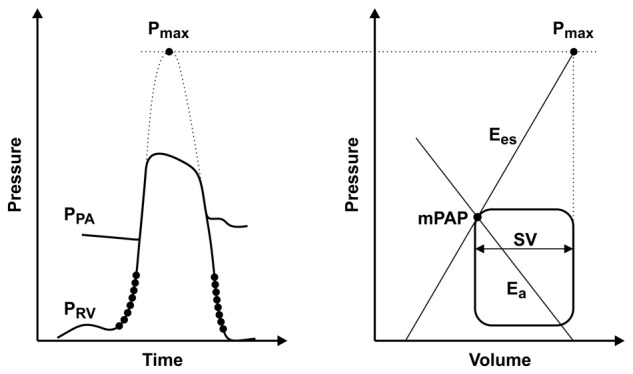
Prediction of right ventricular–to–pulmonary vascular (RV-PV) coupling by the single-beat method. Left, maximal ventricular pressure (Pmax) as encountered in an isovolumetric nonejecting beat, which is estimated by fitting a sinus wave over a right ventricular pressure (PRV) curve during the isovolumetric contraction-and-relaxation phase. PPA: pulmonary artery pressure. Right, end-systolic ventricular elastance (Ees) can be derived from Pmax by dividing the difference between Pmax and mean pulmonary artery pressure (mPAP) by the stroke volume (SV). Arterial elastance (Ea) can be estimated as mPAP/SV. Adapted from Trip et al.17
Noninvasive assessment of RV afterload and function
Assessment of RV function by imaging is challenging because of its complex anatomy. While RV ejection fraction (RVEF) is commonly used as an index of RV function, it is highly load dependent and does not reflect RV contractility.24 This problem affects the clinical assessment and management of patients with RV dysfunction, including those with acute and chronic PE. On that account, other parameters reflecting RV function, such as RV fractional area change (RVFAC) and tricuspid annular plane systolic excursion (TAPSE), have been introduced into clinical routine.25 More recently, several indexes have been proposed as surrogates of RV function and contractility, including the RV myocardial performance index (RVMPI, or Tei index)26-28 and the acceleration of the myocardium during isovolumetric contraction (IVA).29 Based on Doppler imaging, RVMPI is an established marker of myocardial performance and ventricular contractility that is independent of ventricular geometry. RVMPI has been evaluated for the assessment of LV and RV function in heart failure and PH.26-28,30-33 Myocardial deformation parameters of the RV free wall, such as strain and strain rate, have been proposed as load- and heart motion–independent measures of RV function.34-36 It has been suggested that echocardiographic indexes of RV function, including TAPSE, RVMPI, RVFAC, and IVA, are related to RV-PV coupling (Ees/Ea) rather than to Ees alone.19 RV echocardiographic indexes, especially RVFAC and IVA, have been shown to correlate more strongly with Ees/Ea than with Ees in an experimental model of RV chronic pressure overload induced by pulmonary arterial ligation and recurrent embolization.19
RV-PV coupling can also be determined by combining standard right heart catheterization and measurements derived during magnetic resonance imaging. Studies in healthy individuals and patients with PH have shown a good agreement with conductance catheterization data.20,21
The RV in acute PE
Pathogenesis and epidemiology
Acute PE and DVT are part of the spectrum of VTE. Thrombi commonly form in deep veins in the legs. Venous thrombi detach from their formation sites and embolize through the venous system, right atrium, and RV toward the pulmonary circulation. DVT has an incidence of 1.5 per 1,000 person-years. About 79% of patients who present with PE have evidence of DVT. One-half of the patients with proximal DVT experience an episode of PE. The direct consequence of PE is an elevation in RV afterload, followed by an increase in RV wall tension that may lead to dilatation, dysfunction, and ischemia of the RV. Death results from RV failure. RV ischemia in acute PE results from myocyte necrosis and myocardial inflammation by infiltration with macrophages, T cells, and neutrophils and is distinct from the pattern due to epicardial vessel occlusion seen in myocardial infarction.37,38
Noninvasive assessment and risk stratification of acute PE
Electrocardiographic signs of RV strain, such as T wave inversions in V1–V4, QR pattern in V1, the S1Q3T3 pattern, and incomplete or complete right bundle-branch block, are useful but insensitive for the assessment of RV dysfunction in acute PE. However, the presence of RV strain on electrocardiogram has been shown to correlate with the extent of pulmonary vascular obstruction39 and outcomes of acute PE.40 At least 25% of patients with acute PE have signs of RV dysfunction on echocardiography. Overall and in-hospital mortality rates have been shown to be higher in patients with echocardiographic signs of RV dysfunction at the time of diagnosis of acute PE.41-44 Patients with systolic pulmonary artery pressures higher than 50 mmHg estimated by echocardiography at the time of diagnosis have a 3-fold risk for persistent PH and RV dysfunction.45
RV afterload in acute PE
Acute PE leads to a number of pathophysiological changes in pulmonary function. The most important among these alterations is an acute increase in PVR. Abrupt increase in PVR leading to RV failure is the principle cause of death from PE. The mPAP and PVR increase in proportion to the degree of pulmonary vascular obstruction in patients without preexisting pulmonary vascular disease.46,47 In a series of 76 patients with acute PE and without previous cardiopulmonary disease, long-term prognosis was related to the level of mPAP and the presence of RV failure.1 Patients with mPAP higher than 30 mmHg at initial diagnosis had progressive PH. Survival after 2 years of follow-up was less than 20% when mPAP was higher than 50 mmHg.1
In contrast, acute PE in patients with preexisting pulmonary vascular disease leads to higher pulmonary artery pressures as a result of RV hypertrophy. It has been shown that there is no correlation between the degree of pulmonary vascular obstruction and mPAP in patients with acute PE superimposed on pulmonary vascular disease.47 The diastolic pulmonary vascular pressure gradient (DPG), i.e., the difference between diastolic pulmonary artery pressure and mean pulmonary arterial wedge pressure [mPAWP]) has been described as elevated in acute PE.48 A positive correlation has been found between DPG and the percentage of embolic pulmonary arterial tree obstruction, as assessed by pulmonary angiography.49-51 Furthermore, DPG has been shown to be useful for the differentiation between pulmonary and cardiac causes of acute respiratory failure52 and PH.53-55
RV function in acute PE
The extent of pulmonary vascular obstruction and the presence of preexisting cardiopulmonary disease determine the increase in RV afterload and the development of RV dysfunction.56 The sudden rises in pulmonary artery pressure and PVR abruptly increase RV afterload, consequently leading to an increase in RV wall tension, followed by RV dilation and dysfunction. As the RV dilates, the interventricular septum shifts toward the LV. Progressive RV dilation accompanied by LV compression results in diastolic dysfunction and underfilling of the LV. LV underfilling leads to a decrease in systemic cardiac output and systemic blood pressure, potentially impairing coronary perfusion. Elevated RV wall tension itself reduces right coronary artery flow, increases RV myocardial oxygen consumption and demand, and causes ischemia.57
The RV in CTPVD/CTEPH
Pathogenesis and epidemiology
Incomplete resolution of acute PE is frequently observed after acute PE but rarely results in CTEPH. Some symptomatic patients may present with normal pulmonary hemodynamics at rest, despite symptomatic disease, e.g., patients with complete unilateral obstruction. Despite the absence of PH at rest, they are treated as CTEPH patients. Suitable terminology to describe this condition of CTPVD, or chronic PE, has not been accepted. In previous studies, the cumulative incidence of CTEPH after acute PE was reported as 0.1%–9.1% after symptomatic PE.45,58-66 However, one has to take into account that the initial thromboembolic event may have been asymptomatic. For example, in the recent European CTEPH registry, 28% of patients did not have a history of acute VTE.67 Therefore, the true incidence of CTEPH may be even higher. Pulmonary endarterectomy (PEA) is the treatment of choice for CTEPH,68 with a periprocedural mortality rate of less than 5% in Europe today,69 nearly normalized hemodynamics, and substantial improvement in clinical symptoms in the majority of patients.68-70
Diagnosis and definition
A diagnosis of CTEPH can be made only after effective anticoagulation of at least 3 months to discriminate the condition from subacute PE.71 CTEPH is defined by an mPAP of at least 25 mmHg with mPAWP no higher than 15 mmHg in the presence of at least one (segmental) perfusion defect detected by lung scanning/multidetector computed tomographic angiography or pulmonary angiography.
RV afterload in CTEPH
Chronic obstructions in the pulmonary circulation lead to an increase in RV afterload and eventually to an impairment in RV function. RV afterload is characterized by a steady flow component, expressed by PVR, an oscillatory load component, expressed by CPA, and characteristic impedance (Z) of the proximal pulmonary artery.2 Despite similar PVRs, mPAP is lower in CTEPH than in iPAH,72 which has led to the assumption that RV adaptation may be poorer in CTEPH patients than in iPAH patients.73 This observation may be explained by the generally older age of patients, the long duration of functional impairment, and disease-specific alterations of the pulmonary circulation.73 RC-time is significantly lower in proximal CTEPH than in distal CTEPH and PAH, which can be explained by structural differences of the pulmonary circulation and implies a higher RV workload.74 Compliance (CPA) has been shown to be of greater prognostic importance than resistance in patients with iPAH.5,6 The prognostic value of CPA could also be demonstrated in CTEPH patients undergoing PEA.75 In a study of 110 consecutive patients76 assessing RV afterload in CTEPH patients before PEA, immediately after PEA, and 1 year after PEA, 84% of the patients had an immediate improvement in CPA after PEA. Immediate postoperative CPA was identified as a univariate predictor of outcome. However, in multivariate analysis, immediate postoperative PVR was the only independent predictor of long-term survival/freedom from lung transplantation after PEA. Patients who had concurrent improvements in CPA and PVR had the lowest likelihood for adverse outcomes (death or persistent/recurrent PH) after PEA (Fig. 2).76 According to current knowledge, PVR appears to be the most critical measure in CTEPH patients, especially when measured immediately after PEA.
Figure 2.
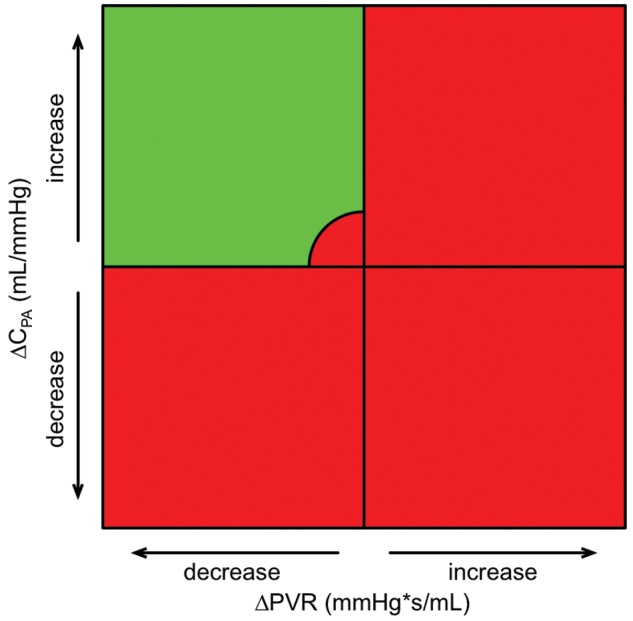
Quadrants summarizing vectors that depict changes in both pulmonary vascular resistance (PVR) and pulmonary arterial compliance (CPA) between catheterizations at baseline and immediately after pulmonary endarterectomy, to discriminate “PVR and CPA responders” from “PVR and CPA nonresponders.” All patients’ vectors with persistent/recurrent pulmonary hypertension in the upper-left quadrant are localized within the circled area (representing cases with only a minor improvement in CPA and PVR). Hemodynamic changes that are associated with improved survival are all within the green quadrant.
PEA has been shown to lead to an immediate decrease in PVR and a concordant increase in CPA,76 resulting in a mild decrease in RC-time (baseline: 0.72 ± 0.71 seconds, immediately after PEA: 0.60 ± 0.3 seconds; P = 0.13). A significant decrease in RC-time after PEA was reported in a study from the United Kingdom (baseline: 0.49 ± 0.11 seconds, after PEA: 0.38 ± 0.11 seconds; P < 0.05).77 It appears that patients from whom more and longer thrombus material can be removed have a better improvement in hemodynamics and are less likely to develop persistent/recurrent PH.76 Specimen assessment takes into account both the technical success of PEA and the anatomical CTEPH type that has previously been correlated with outcomes.78 It has been reported that patients with persistent exertional dyspnea after successful PEA display an abnormal pulmonary hemodynamic response to exercise, characterized by increased PVR and decreased CPA.3
Upstream resistance is significantly higher in operable CTEPH than in nonoperable CTEPH and iPAH.79 Patients with higher downstream and lower upstream resistance before PEA appear to be at increased risk for persistent PH and poor outcomes after PEA.80 Data indicate that patients with upstream resistance of less than 60% are at highest risk for adverse outcomes after PEA.80 It has been suggested that CTEPH patients with lower upstream and higher downstream resistance may suffer from concomitant small-vessel disease that resembles PAH, but this has not been proven by histologic evidence.
RV function in CTPVD/CTEPH
Changes in pressure-volume loop morphology have been observed in CTEPH patients as well as in patients with CTPVD.23 Compared to healthy controls, patients with CTEPH and CTPVD display a positive pressure differential during systolic ejection from pulmonary valve opening to valve closure (−6.3 ± 3.0, 3.2 ± 3.5, and 20.4 ± 18.4 mmHg for controls, CTPVD patients, and CTEPH patients, respectively).23 The RV has been shown to be decoupled in patients with CTEPH, with an Ees/Ea ratio of 0.6 ± 0.18, compared to controls (Ees/Ea = 1.46 ± 0.3). RV-PV decoupling could be observed in patients with CTPVD (Ees/Ea = 1.27 ± 0.36), suggesting RV dysfunction despite normal hemodynamics. In addition, slower RV relaxation, as indicated by the time constant of isovolumetric relaxation τ, has been found in CTEPH and CTPVD patients, compared to controls.23
RV remodeling in CTEPH and reverse remodeling after PEA
As a consequence of chronic increase in afterload, the RV undergoes a remodeling process in order to maintain pulmonary blood flow. This process is characterized by an increase in wall thickness and chamber dilatation. According to the Laplace relationship, the thin-walled RV augments thickness in order to cope with a chronic increase in intraluminal pressure and wall stress.81 This is mainly achieved by hypertrophy. With dilatation, the RV loses its triangular shape, leftward ventricular septal bowing develops, and tricuspid annular dilation results in tricuspid regurgitation. Leftward ventricular septal bowing with LV compression, in combination with low LV preload and underfilling (“LV unloading”), has been shown to cause LV diastolic filling impairment in a significant proportion of patients with CTEPH.82 Chronic LV unloading leads to atrophic remodeling, with LV diastolic and systolic dysfunction.83 A recent study has shown that RV failure in CTEPH patients is associated with a reduction in LV free-wall mass, which is reversible after PEA.83 In addition, the authors showed, in an animal model, that this observation might be explained by myocyte shrinkage due to atrophic remodeling.83 PEA has been shown to restore RV remodeling by an acute reduction in RV afterload,84,85 with subsequent improvement in biventricular cardiac function and reduction in ventricular septal abnormalities and RV systolic wall stress.86,87 Magnetic resonance imaging studies have shown that reverse remodeling occurs early after PEA.86 Echocardiographic studies demonstrated improvements in RV volumes and areas,88 tricuspid regurgitation,89,90 TAPSE,85 RV strain,35 and RVMPI,30,85 indicating better RV function after PEA. Reductions in RV mass and septal bowing, accompanied by an increase in RVEF, have been documented by magnetic resonance imaging studies.84,91 Moreover, the magnitude of reverse RV remodeling after PEA has been shown to correlate with changes in hemodynamics.84,85 However, despite significant improvement in RV function after PEA, RVEF remained reduced compared to that in healthy individuals.84,85 The reason why indexes of RV function, including RVEF, RVMPI, and TAPSE, fail to completely recover after PEA is unknown. It has been suggested that RV remodeling is only partly reversible because of diffuse myocardial fibrosis, similar to PAH.12
Outlook
Mortality in acute PE and CTEPH depends on RV function. One may assume that, in contrast to that in iPAH and scleroderma-associated PAH, the RV in VTE is intrinsically normal, making VTE-dependent RV function an ideal model to study consequences of abrupt (acute PE) and gradual (CTEPH) afterload increase, because RV functional changes are correlated with the degree of mechanical obstruction of the pulmonary circulation. RV functional recovery after PEA in CTEPH is a further attractive human model to study reverse RV remodeling that may provide insights in RV adaptation to increased afterload.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81(2):151–158. [DOI] [PubMed]
- 2.Lankhaar J-W, Westerhof N, Faes TJ, Marques KMJ, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 2006;291(4):H1731–H1737. [DOI] [PubMed]
- 3.Bonderman D, Martischnig AM, Vonbank K, Nikfardjam M, Meyer B, Heinz G, Klepetko W, Naeije R, Lang IM. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest 2011;139(1):122–127. [DOI] [PubMed]
- 4.Lankhaar JW, Westerhof N, Faes TJ, Gan CT-J, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J 2008;29(13):1688–1695. [DOI] [PubMed]
- 5.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006;47(4):799–803. [DOI] [PubMed]
- 6.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. J Am Coll Cardiol Cardiovasc Imaging 2012;5(4):378–387. [DOI] [PubMed]
- 7.Hakim TS, Michel RP, Chang HK. Partitioning of pulmonary vascular resistance in dogs by arterial and venous occlusion. J Appl Physiol 1982;52(3):710–715. [DOI] [PubMed]
- 8.Kafi SA, Melot C, Vachiéry JL, Brimioulle S, Naeije R. Partitioning of pulmonary vascular resistance in primary pulmonary hypertension. J Am Coll Cardiol 1998;31(6):1372–1376. [DOI] [PubMed]
- 9.Fesler P, Pagnamenta A, Vachiéry JL, Abdel Kafi S, Boonstra A, Delcroix M, Channick RN, Rubin LJ, Naeije R. Single arterial occlusion to locate resistance in patients with pulmonary hypertension. Eur Respir J 2003;21(1):31–36. [DOI] [PubMed]
- 10.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 2005;289(2):H501–H512. [DOI] [PubMed]
- 11.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 1976;58(3):751–760. [DOI] [PMC free article] [PubMed]
- 12.Rain S, Handoko ML, Trip P, Gan CT-J, Westerhof N, Stienen GJ, Paulus WJ, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013;128(18):2016–2025. [DOI] [PubMed]
- 13.Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. New York: Oxford University Press, 1988.
- 14.Vonk-Noordegraaf A, Westerhof N. Describing right ventricular function. Eur Respir J 2013;41(6):1419–1423. [DOI] [PubMed]
- 15.Sunagawa K, Yamada A, Senda Y, Kikuchi Y, Nakamura M, Shibahara T, Nose Y. Estimation of the hydromotive source pressure from ejecting beats of the left ventricle. IEEE (Inst Electr Electron Eng) Trans Biomed Eng 1980;27(6):299–305. [DOI] [PubMed]
- 16.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003;284(5):H1625–H1630. [DOI] [PubMed]
- 17.Trip P, Kind T, van de Veerdonk MC, Marcus JT, de Man FS, Westerhof N, Vonk-Noordegraaf A. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 2013;32(1):50–55. [DOI] [PubMed]
- 18.Wauthy P, Pagnamenta A, Vassalli F, Naeije R, Brimioulle S. Right ventricular adaptation to pulmonary hypertension: an interspecies comparison. Am J Physiol Heart Circ Physiol 2004;286(4):H1441–H1447. [DOI] [PubMed]
- 19.Guihaire J, Haddad F, Boulate D, Decante B, Denault AY, Wu J, Hervé P, et al. Non-invasive indices of right ventricular function are markers of ventricular-arterial coupling rather than ventricular contractility: insights from a porcine model of chronic pressure overload. Eur Heart J Cardiovasc Imaging 2013;14(12):1140–1149. [DOI] [PubMed]
- 20.Kuehne T, Yilmaz S, Steendijk P, Moore P, Groenink M, Saaed M, Weber O, et al. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation 2004;110(14):2010–2016. [DOI] [PubMed]
- 21.Sanz J, Garcia-Alvarez A, Fernández-Friera L, Nair A, Mirelis JG, Sawit ST, Pinney S, Fuster V. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012;98(3):238–243. [DOI] [PubMed]
- 22.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013;6(5):953–963. [DOI] [PMC free article] [PubMed]
- 23.McCabe C, White PA, Hoole SP, Axell RG, Priest AN, Gopalan D, Taboada D, et al. Right ventricular dysfunction in chronic thromboembolic obstruction of the pulmonary artery. J Appl Physiol 2014;116(4):355–363. doi:10.1152/japplphysiol.01123.2013. [DOI] [PMC free article] [PubMed]
- 24.Helbing WA, Bosch HG, Maliepaard C, Rebergen SA, van der Geest RJ, Hansen B, Ottenkamp J, Reiber JHC, de Roos A. Comparison of echocardiographic methods with magnetic resonance imaging for assessment of right ventricular function in children. Am J Cardiol 1995;76(8):589–594. [DOI] [PubMed]
- 25.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23(7):685–713. [DOI] [PubMed]
- 26.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward JB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996;9(6):838–847. [DOI] [PubMed]
- 27.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol 1998;81(9):1157–1161. [DOI] [PubMed]
- 28.Vonk MC, Sander MH, van den Hoogen FH, van Riel PL, Verheugt FW, van Dijk AP. Right ventricle Tei-index: a tool to increase the accuracy of non-invasive detection of pulmonary arterial hypertension in connective tissue diseases. Eur J Echocardiogr 2007;8(5):317–321. [DOI] [PubMed]
- 29.Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, Redington AN. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 2002;105(14):1693–1699. [DOI] [PubMed]
- 30.Blanchard DG, Malouf PJ, Gurudevan SV, Auger WR, Madani MM, Thistlethwaite P, Waltman TJ, Daniels LB, Raisinghani AB, DeMaria AN. Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. J Am Coll Cardiol Cardiovasc Imaging 2009;2(2):143–149. [DOI] [PubMed]
- 31.Dujardin KS, Tei C, Yeo TC, Hodge DO, Rossi A, Seward JB. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 1998;82(9):1071–1076. [DOI] [PubMed]
- 32.Eidem BW, Tei C, O’Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr 1998;11(9):849–856. [DOI] [PubMed]
- 33.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol 1995;26(6):357–366. [PubMed]
- 34.Dambrauskaite V, Delcroix M, Claus P, Herbots L, D’hooge J, Bijnens B, Rademakers F, Sutherland GR. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 2007;20(10):1172–1180. [DOI] [PubMed]
- 35.Giusca S, Dambrauskaite V, Scheurwegs C, D’hooge J, Claus P, Herbots L, Magro M, et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart 2010;96(4):281–288. [DOI] [PubMed]
- 36.Huez S, Vachiéry JL, Unger P, Brimioulle S, Naeije R. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol 2007;100(9):1473–1478. [DOI] [PubMed]
- 37.Orde MM, Puranik R, Morrow PL, Duflou J. Myocardial pathology in pulmonary thromboembolism. Heart 2011;97(20):1695–1699. [DOI] [PubMed]
- 38.Watts JA, Zagorski J, Gellar MA, Stevinson BG, Kline JA. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 2006;41(2):296–307. [DOI] [PubMed]
- 39.Kjaergaard J, Schaadt BK, Lund JO, Hassager C. Quantification of right ventricular function in acute pulmonary embolism: relation to extent of pulmonary perfusion defects. Eur J Echocardiogr 2008;9(5):641–645. [DOI] [PubMed]
- 40.Stergiopoulos K, Bahrainy S, Strachan P, Kort S. Right ventricular strain rate predicts clinical outcomes in patients with acute pulmonary embolism. Acute Card Care 2011;13(3):181–188. [DOI] [PubMed]
- 41.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 1997;134(3):479–487. [DOI] [PubMed]
- 42.Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care 2011;15:R103. [DOI] [PMC free article] [PubMed]
- 43.Becattini C, Agnelli G, Vedovati MC, Pruszczyk P, Casazza F, Grifoni S, Salvi A, et al. Multidetector computed tomography for acute pulmonary embolism: diagnosis and risk stratification in a single test. Eur Heart J 2011;32(13):1657–1663. [DOI] [PubMed]
- 44.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007;116(4):427–433. [DOI] [PubMed]
- 45.Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography Doppler and five-year survival analysis. Circulation 1999;99(10):1325–1330. [DOI] [PubMed]
- 46.Delcroix M, Melot C, Vachiéry JL, Lejeune P, Leeman M, Vanderhoeft P, Naeije R. Effects of embolus size on hemodynamics and gas exchange in canine embolic pulmonary hypertension. J Appl Physiol 1990;69(6):2254–2261. [DOI] [PubMed]
- 47.Sharma GV, McIntyre KM, Sharma S, Sasahara AA. Clinical and hemodynamic correlates in pulmonary embolism. Clin Chest Med 1984;5(3):421–437. [PubMed]
- 48.Buchbinder N, Ganz W. Hemodynamic monitoring: invasive techniques. Anesthesiology 1976;45(2):146–155. [DOI] [PubMed]
- 49.McIntyre KM, Sasahara AA. The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol 1971;28(3):288–294. [DOI] [PubMed]
- 50.McIntyre KM, Sasahara AA. Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest 1974;65(5):534–543. [DOI] [PubMed]
- 51.Enson Y. Pulmonary heart disease: relation of pulmonary hypertension to abnormal lung structure and function. Bull NY Acad Med 1977;53(6):551–566. [PMC free article] [PubMed]
- 52.Stevens PM. Assessment of acute respiratory failure: cardiac versus pulmonary causes. Chest 1975;67(1):1–2. [DOI] [PubMed]
- 53.Harvey RM, Enson Y, Ferrer MI. A reconsideration of the origins of pulmonary hypertension. Chest 1971;59(1):82–94. [DOI] [PubMed]
- 54.Naeije R, Vachiéry JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013;41(1):217–223. [DOI] [PubMed]
- 55.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013;143(3):758–766. [DOI] [PubMed]
- 56.Konstantinides S. Pulmonary embolism: impact of right ventricular dysfunction. Curr Opin Cardiol 2005;20(6):496–501. [DOI] [PubMed]
- 57.Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation 2005;112(2):e28–32. [DOI] [PubMed]
- 58.Surie S, Gibson NS, Gerdes VEA, Bouma BJ, van Eck-Smit BLF, Buller HR, Bresser P. Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb Res 2010;125(5):e202–e205. [DOI] [PubMed]
- 59.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. New Engl J Med 2001;345(20):1465–1472. [DOI] [PubMed]
- 60.Benotti JR, Ockene IS, Alpert JS, Dalen JE. The clinical profile of unresolved pulmonary embolism. Chest 1983;84(6):669–678. [DOI] [PubMed]
- 61.Dentali F, Donadini M, Gianni M, Bertolini A, Squizzato A, Venco A, Ageno W. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009;124(3):256–258. [DOI] [PubMed]
- 62.Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, Ageno W. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130(1):172–175. [DOI] [PubMed]
- 63.Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350(22):2257–2264. [DOI] [PubMed]
- 64.Martí D, Gómez V, Escobar C, Wagner C, Zamarro C, Sánchez D, Sam A, et al. Incidence of symptomatic and asymptomatic chronic thromboembolic pulmonary hypertension [in Spanish]. Arch Bronconeumol 2010;46(12):628–633. [DOI] [PubMed]
- 65.Klok FA, van Kralingen KW, van Dijk APJ, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010;95(6):970–975. [DOI] [PMC free article] [PubMed]
- 66.Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, Dainelli A, Giuntini C. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine 2006;85(5):253–262. [DOI] [PubMed]
- 67.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambrož D, Treacy C, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124(18):1973–1981. [DOI] [PubMed]
- 68.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76(5):1457–1464. [DOI] [PubMed]
- 69.Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141(3):702–710. [DOI] [PubMed]
- 70.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg 2008;14(5):274–282. [PubMed]
- 71.Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990;81(6):1735–1743. [DOI] [PubMed]
- 72.Lang IM, Simonneau G, Pepke-Zaba JW, Mayer E, Ambrož D, Blanco I, Torbicki A, Mellemkjaer S, Yaïci A, Delcroix M. Factors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension: a case-control study. Thromb Haemostasis 2013;110(1):83–91. [DOI] [PubMed]
- 73.Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J 2013;41(1):224–232. [DOI] [PubMed]
- 74.Pagnamenta A, Vanderpool R, Brimioulle S, Naeije R. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol 2013;114(11):1586–1592. [DOI] [PubMed]
- 75.de Perrot M, McRae K, Shargall Y, Thenganatt J, Moric J, Mak S, Granton JT. Early postoperative pulmonary vascular compliance predicts outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Chest 2011;140(1):34–41. [DOI] [PubMed]
- 76.Skoro-Sajer N, Marta G, Gerges C, Hlavin G, Nierlich P, Taghavi S, Sadushi-Kolici R, Klepetko W, Lang IM. Surgical specimens, haemodynamics and long-term outcomes after pulmonary endarterectomy. Thorax 2014;69(2):116–122. [DOI] [PMC free article] [PubMed]
- 77.MacKenzie Ross RV, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2013;305(2):H259–H264. [DOI] [PMC free article] [PubMed]
- 78.Thistlethwaite PA, Mo M, Madani MM, Deutsch R, Blanchard D, Kapelanski DP, Jamieson SW. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2002;124(6):1203–1211. [DOI] [PubMed]
- 79.Toshner M, Suntharalingam J, Fesler P, Soon E, Sheares KK, Jenkins D, White P, Morrell NW, Naeije R, Pepke-Zaba J. Occlusion pressure analysis role in partitioning of pulmonary vascular resistance in CTEPH. Eur Respir J 2012;40(3):612–617. [DOI] [PubMed]
- 80.Kim NH, Fesler P, Channick RN, Knowlton KU, Ben-Yehuda O, Lee SH, Naeije R, Rubin LJ. Preoperative partitioning of pulmonary vascular resistance correlates with early outcome after thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Circulation 2004;109(1):18–22. [DOI] [PubMed]
- 81.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135(3):794–804. [DOI] [PubMed]
- 82.Gurudevan SV, Malouf PJ, Auger WR, Waltman TJ, Madani M, Raisinghani AB, DeMaria AN, Blanchard DG. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol 2007;49(12):1334–1339. [DOI] [PubMed]
- 83.Hardziyenka M, Campian ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: evidence for atrophic remodeling. J Am Coll Cardiol 2011;57(8):921–928. [DOI] [PubMed]
- 84.Reesink HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk Noordegraaf A, Bresser P. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg 2007;133(1):58–64. [DOI] [PubMed]
- 85.Surie S, Bouma BJ, Bruin-Bon RA, Hardziyenka M, Kloek JJ, Van der Plas MN, Reesink HJ, Bresser P. Time course of restoration of systolic and diastolic right ventricular function after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Am Heart J 2011;161(6):1046–1052. [DOI] [PubMed]
- 86.Iino M, Dymarkowski S, Chaothawee L, Delcroix M, Bogaert J. Time course of reversed cardiac remodeling after pulmonary endarterectomy in patients with chronic pulmonary thromboembolism. Eur Radiol 2008;18(4):792–799. [DOI] [PubMed]
- 87.Mauritz G-J, Vonk-Noordegraaf A, Kind T, Surie S, Kloek JJ, Bresser P, Saouti N, Bosboom J, Westerhof N, Marcus JT. Pulmonary endarterectomy normalizes interventricular dyssynchrony and right ventricular systolic wall stress. J Cardiovasc Magn Reson 2012;14:5. [DOI] [PMC free article] [PubMed]
- 88.Menzel T, Kramm T, Brückner A, Mohr-Kahaly S, Mayer E, Meyer J. Quantitative assessment of right ventricular volumes in severe chronic thromboembolic pulmonary hypertension using transthoracic three-dimensional echocardiography: changes due to pulmonary thromboendarterectomy. Eur J Echocardiogr 2002;3(1):67–72. [DOI] [PubMed]
- 89.Li YD, Zhai ZG, Wu YF, Yang YH, Gu S, Liu Y, Su PX, Wang C. Improvement of right ventricular dysfunction after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of echocardiography to demonstrate restoration of the right ventricle during 2-year follow-up. Thromb Res 2013;131(5):e196–201. [DOI] [PubMed]
- 90.Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg 2012;94(1):97–103. [DOI] [PubMed]
- 91.Kreitner K-F, Ley S, Kauczor H-U, Mayer E, Kramm T, Pitton MB, Krummenauer F, Thelen M. Chronic thromboembolic pulmonary hypertension: pre- and postoperative assessment with breath-hold MR imaging techniques. Radiology 2004;232(2):535–543. [DOI] [PubMed]


