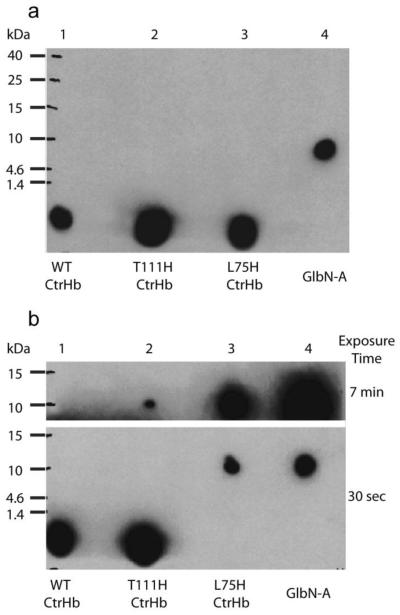
Fig. 4.
ECL SDS-PAGE detection of covalently bound heme in CtrHbs. (a) Ferric samples were reduced with 2 mM DT for ~30 min then re-oxidized with air before being subjected to electrophoresis. The image was obtained with a 30-s exposure. The heme of WT CtrHb and the T111H and L75H variants (lane 1-3) migrates at the dye front. WT GlbN-A (lane 4) is included as a positive control for covalent linkage. (b) Ferric WT and variant CtrHbs were first saturated with cyanide and then reduced for ~30 min. The figure shows images of film that was exposed for either 30 s or 7 min. The heme from WT CtrHb (lane 1) migrates with the dye front. The T111H CtrHb lane (lane 2) shows incomplete heme attachment. The longer exposure time was necessary to observe the faint crosslinked T111H CtrHb band. The L75H CtrHb lane (lane 3) shows complete crosslinking as in the GlbN-A control (lane 4).