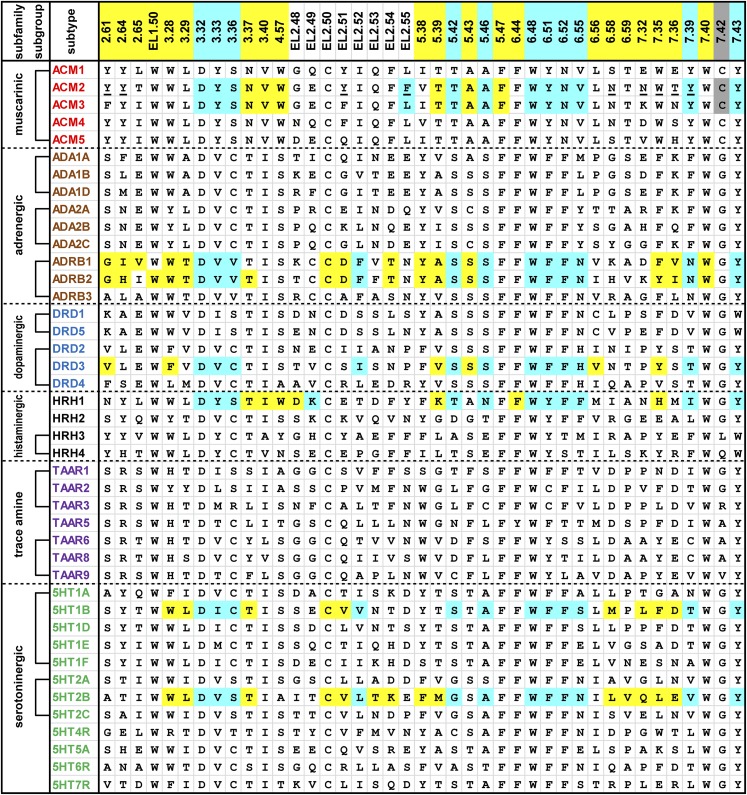
Fig. 1.
Alignment of the ligand-contacting residues in the 42 human aminergic receptors. An analysis of ligand-contacting residues was carried out for all 36 available ligand-bound crystal structures of aminergic receptors. The residue positions are indicated by the Ballesteros-Weinstein numbers at the top of each column (for EL2, the residues are indexed relative to the conserved Cys residue, EL2.50, which makes a disulfide bond to Cys3.25) and colored by the classification of the positions: OBS positions (see section II.A) in cyan, SBP positions (see section II.B) in yellow, and 7.42, which contacts ligand but is not in the OBS or any SBP, in gray (see Fig. 2). The OBS positions were defined as the common set of positions identified by the SASA analysis in all structures except for the agonist-bound M2R structures (PDB IDs 4MQS and 4MQT). The SASA values for each residue were calculated using the program Naccess (Hubbard and Thornton, 1993), with probe size of 1.4 Å, Z-slice of 0.001; we used a cutoff of 0.2% to identify the residues with different accessibility in the presence and absence of bound ligand. Note that the OBS includes one residue from EL2 approximately at position EL2.52—although in the H1R structure (PDB ID 3RZE) or the antagonist-bound M2R and M3R structures (PDB IDs 3UON and 4DAJ) EL2.52 does not face the OBS, the residue at EL2.49 or EL2.55 contacts ligand in a manner similar to the residues at EL2.52 in other structures. The receptors are indicated by their UniProt entry names and colored by subfamily. We define a subgroup within a subfamily, indicated by brackets, to be a set of receptors in which all the pairwise sequence identities of the TM domain are >45%. The residues that contact a ligand in any of the structures for a particular receptor are shaded in cyan, yellow, or gray as described above. The residues interacting with the positive allosteric modulator LY2119620 in the M2R structure (PDB ID 4MQT) are underlined, except for Glu172EL2.46 and Ala414EL3, which are not shown. All the amino acid sequences are human; for β1AR and M3R, however, the contact residues were identified in the crystal structures of turkey β1AR and rat M3R.