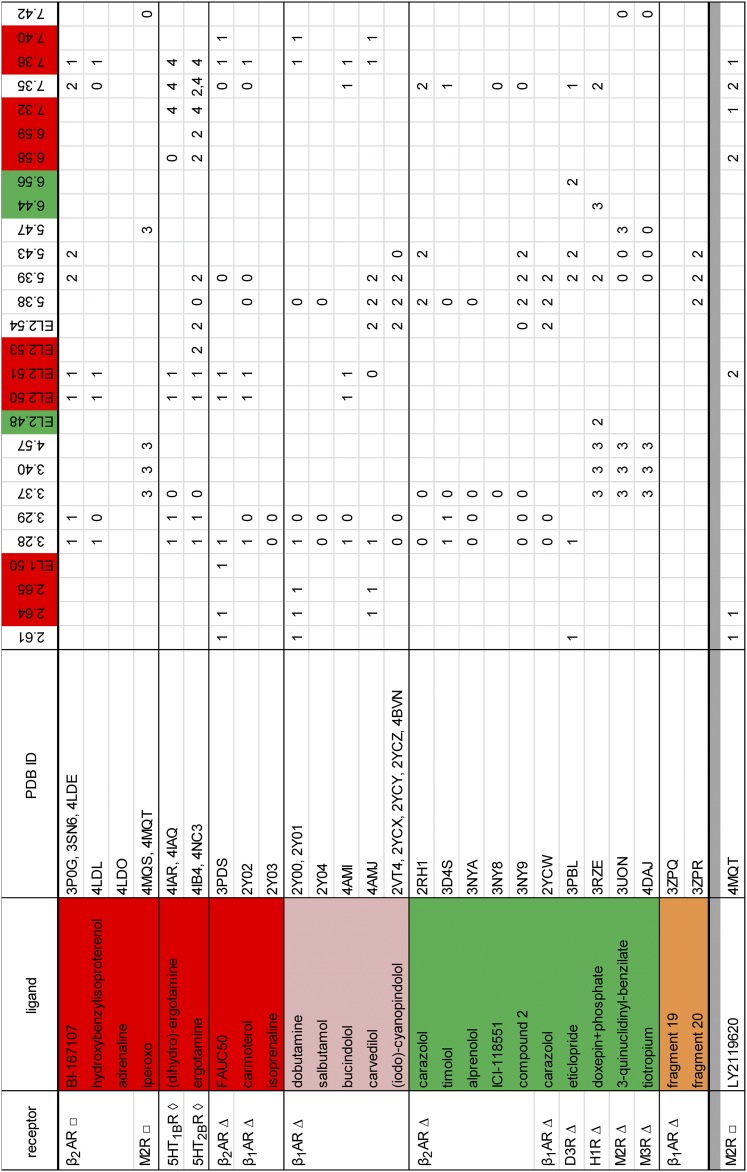
Fig. 2.
Identification of SBPs in the crystal structures of aminergic receptors. The numbered (“0” to “4”) positions for each complex indicate the residues outside of the OBS (non-OBS) that interact with a specific ligand moiety—the closest distance between any heavy atoms of the residue and a ligand moiety is within the van der Waals interaction distance, which we define in this study to be the sum of the van der Waals radii plus 0.8 Å. A SBP was identified as a cluster of ≥3 non-OBS residues that interact with a small ligand moiety, which we defined as a set of ligand heavy atoms with the largest pairwise distance between any pair <4.2 Å (the largest pairwise distance within a hydroxyphenyl moiety). “0” indicates the residue is not within any SBP in that structure, “1” to “4” indicate the involvement of the residue in forming a particular SBP (“1,” “SBP237”; “2,” “SBP567”; “3,” “SBP3456”, “4,” “SBP7”). These SBPs are classified according to their location and named according to the surrounding TMs (see Fig. 4 for the representatives of “1” to “3”); thus, in a particular complex, a ligand moiety does not necessarily interact with every surrounding TM. The structures are arranged according to the conformational state of the receptor (i.e., active, intermediate-active, and inactive, which are indicated by □, ◇, and ∆, respectively) and the ligand efficacy (i.e., agonist in red, partial or biased agonist in pink, and antagonist/inverse agonist in green). The residue sets from the structures for the same receptor bound with the same or highly similar ligand are combined, e.g., the structures with PDB IDs 3P0G, 3SN6, and 4LDE are all β2AR in complex with BI-167107. The fragments bound to β1AR are categorized separately as their efficacies were unknown (colored in orange) (Christopher et al., 2013). The phosphate ion in the H1R structure (PDB ID 3RZE) is counted as an extension of the orthosteric ligand (Shimamura et al., 2011). The positions in the top row are colored if they only interact with either agonists/partial agonists (red) or antagonists/inverse agonists (green).