Fig. 7.
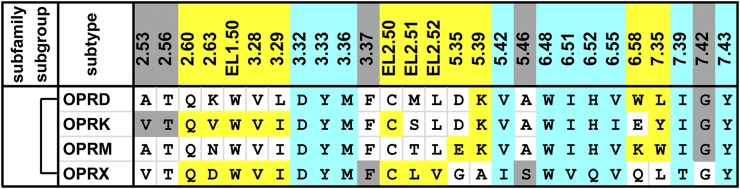
Alignment of the ligand-contacting residues in the four opioid receptors. The ligand-contacting residues were identified by SASA analysis for all five available crystal structures of opioid receptors. The residue positions are indicated by the Ballesteros-Weinstein numbers at the top of each column (for EL2, the residues are indexed relative to the conserved Cys residue, EL2.50, which makes a disulfide bond to Cys3.25) and colored by the classification of the positions: OBS positions in cyan, SBP positions in yellow, and the remaining positions in gray. The OBS and SBP positions were identified similarly to those in the aminergic receptors. Note the identified ligand-contacting residues are similar to those previously summarized (Filizola and Devi, 2013). The bracket on the left indicates high pairwise sequence identities of >60% among all four receptors. The receptors are indicated by their UniProt entry names. All the amino acid sequences are human; for the µ-opioid receptor (OPRM), the contact residues are identified in the crystal structure of mouse OPRM.
