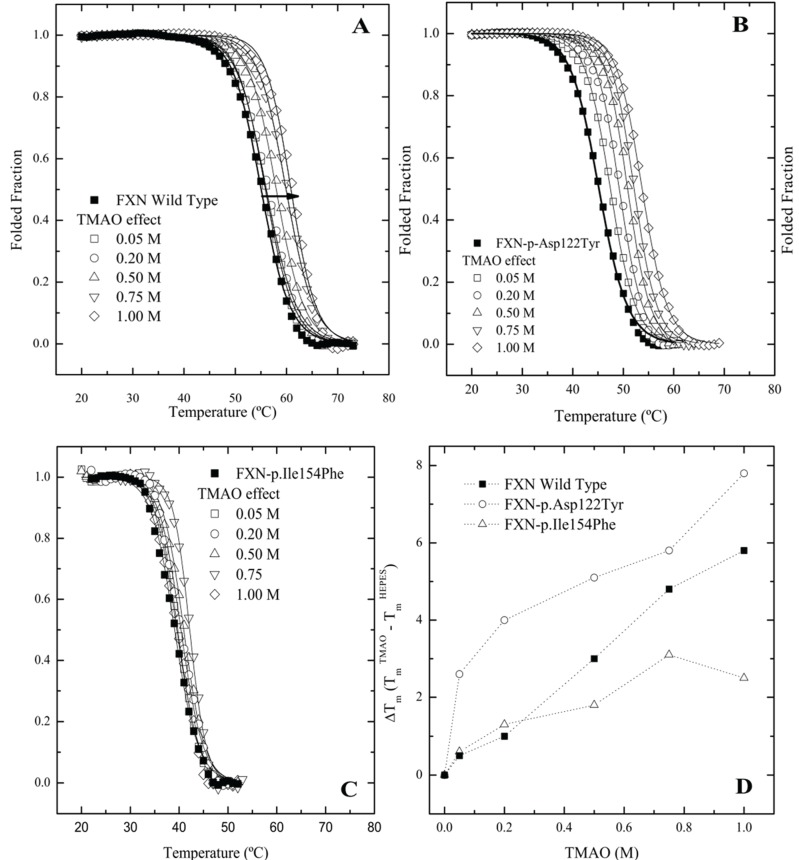
Figure 4.
Differential Scanning fluorimetry (DSF) of native and variant frataxins in the absence and presence of TriMethylAmine N-oxide (TMAO). The concentration of frataxin was 4 µM. The dye Sypro orange was used to probe the temperature dependent protein unfolding reaction. The starting apparent kinetic Tm values without osmolyte were 55.1 °C for wild type FXN (A), 48.8 °C for FXN-p.Asp122Tyr (B) and 39.1 °C for FXN-p.Ile154Phe (C) (see also Table 1). Panels (A–C) Melting curves for the three variants, measured by Differential Scanning Fluorimetry in the presence of increasing concentrations of two osmolytes. Panel (D). TMAO efficiently increases the Tm values of all FXN variants in a concentration dependent way. (■) FXN wild type, (○) FXN-p.Asp122Tyr and (∆) FXN-p.Ile154Phe.