Figure 2.
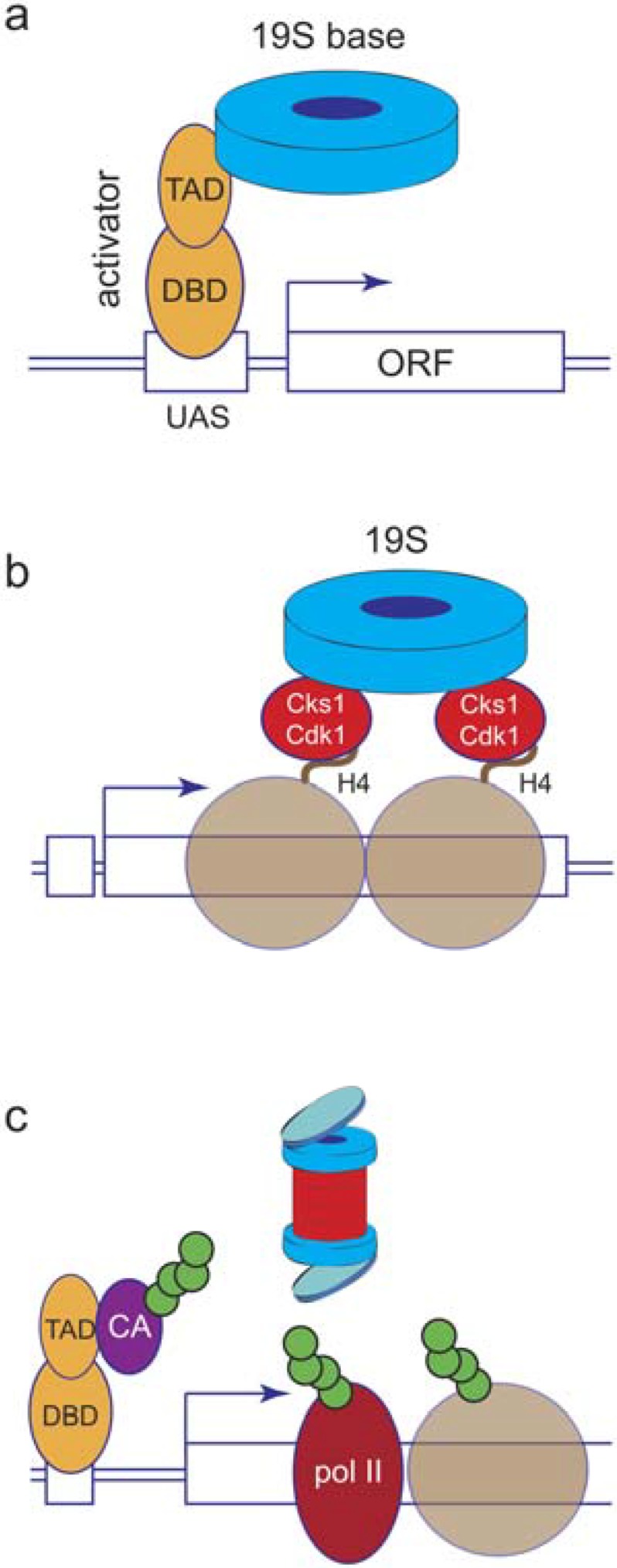
Models for recruitment of proteasome subunits to chromatin. (a) Recruitment by direct contact with activators. A model activator, with its separable DNA-binding domain (DBD) and transcriptional activation domain (TAD) is shown bound to an upstream activating sequence (UAS) at a yeast promoter. In this model, direct contact between the TAD of the activator and one or more ATPases in the 19S base complex leads to promoter-selective recruitment of 19S base components to chromatin; (b) Recruitment by intermediary proteins. In this model, binding of an adapter complex (consisting of Cks1 and Cdk1) to histone H4 (brown circle) tails leads to recruitment of 19S proteasome components; (c) Recruitment by ubiquitylated substrates. In this model, we propose that the presence of one or more ubiquitylated substrates on chromatin recruits proteasomes. “CA” stands for co-activator. Note that this model is consistent with canonical views of how the proteasome is recruited into other biological processes, but the specific ubiquitylated proteins here are arbitrary.
