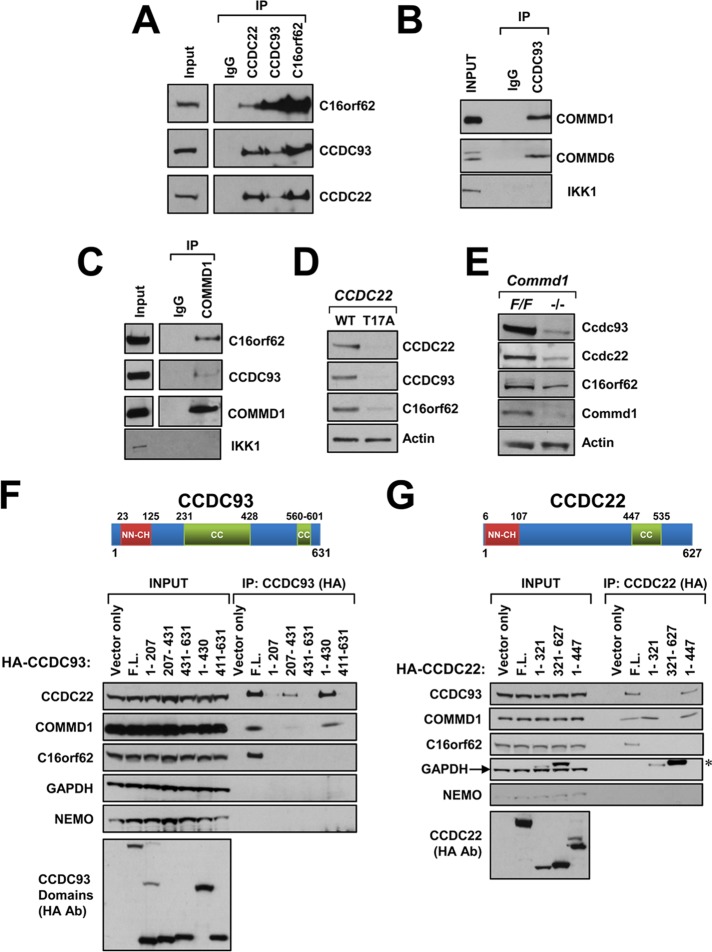
FIGURE 2:
Discovery of the COMMD1–CCDC22 interactome. (A) Coimmunoprecipitation of endogenous CCDC22, CCDC93, and C16orf62 in HEK293T cell lysates confirm their interactions. (B) Endogenous CCDC93 coimmunoprecipitates with COMMD1 and COMMD6 in HEK293T cell lysates. (C) Endogenous COMMD1 coimmunoprecipitates with C16orf62 and CCDC93 in HEK 293 cell lysates. (D) Expression of CCC complex subunits was examined by immunoblotting in CCDC22 T17A fibroblasts. (E) Expression of CCC complex subunits was examined by immunoblotting in Commd1-deficient fibroblasts (Commd1−/−) and its isogenic control (Commd1F/F). (F, G) Domain organization of CCDC93 and CCDC22. Truncation mutants of CCDC93 (F) or CCDC22 (G) were expressed in HEK293T cells and subsequently immunoprecipitated from cell lysates to assess their ability to interact with CCDC22, CCDC93, COMMD1, and C16orf62, as indicated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and NEMO served as specificity controls. The asterisk in G denotes bands resulting from the hemagglutinin (HA) immunoblot and are not the GAPDH band, which migrates faster.