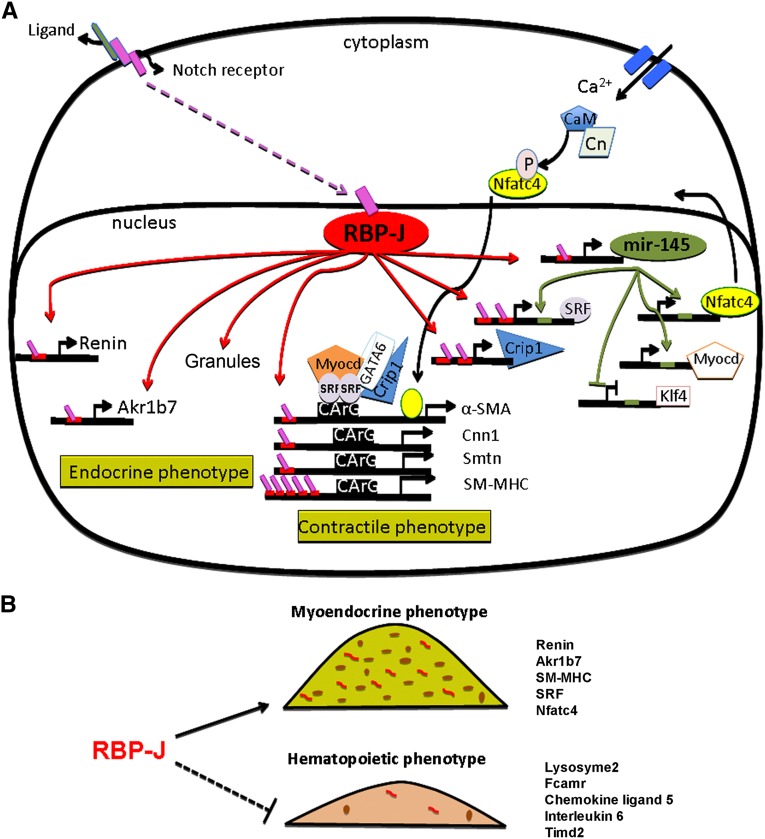
Figure 7.
RBP-J acts as a master regulator that maintains the identity of the JG cell. (A) RBP-J regulates a network of genes that confers the endocrine–contractile phenotype of the JG cell. Red bars located in the promoters of target genes represent RBP-J binding sites. Endocrine genes, such as renin and Akr1b7, and generation of renin granules are indicated on the left side of the diagram. Genes necessary to maintain the contractile phenotype are indicated on the right side. RBP-J regulates the SM genes directly through their promoter regions and indirectly by upregulating the expression of miR-145–5p and SRF. miR-145–5p positively regulates SRF, myocardin, and Nfatc4, and they act together to activate the expression of SM genes.15 SRF binds to CArG sites located in the SM genes. Given that SRF is an miR-145–5p target gene and has two RBP-J sites in its promoter, it is likely that both miR-145–5p and RBP-J regulate the transcriptional activity of SRF. In addition, miR-145–5p also promotes the expression of SM genes by repressing Klf4, a transcription factor that forms a complex with SRF to prevent transcriptional activity of SM genes.46 Crip1, another RBP-J predicted target gene, is an SM marker known to form a complex with GATA 6 and SRF to promote the contractile phenotype. We hypothesize that the canonical Notch signaling pathway is involved in maintaining the myo-endocrine phenotype of the JG cell. The ligand–Notch receptor interaction (yet to be identified) results in the release of the Notch intracellular domain (pink boxes), allowing its translocation to the nucleus, where it binds RBP-J to activate transcription. CaM, calmodulin; Cn, calcineurin; P, phosphate; Smtn, smoothelin. (B) RBP-J maintains the identity of the JG cells by not only activating genes characteristic of their myo-endocrine phenotype but also, preventing the undesirable ectopic expression of genes from other lineages.