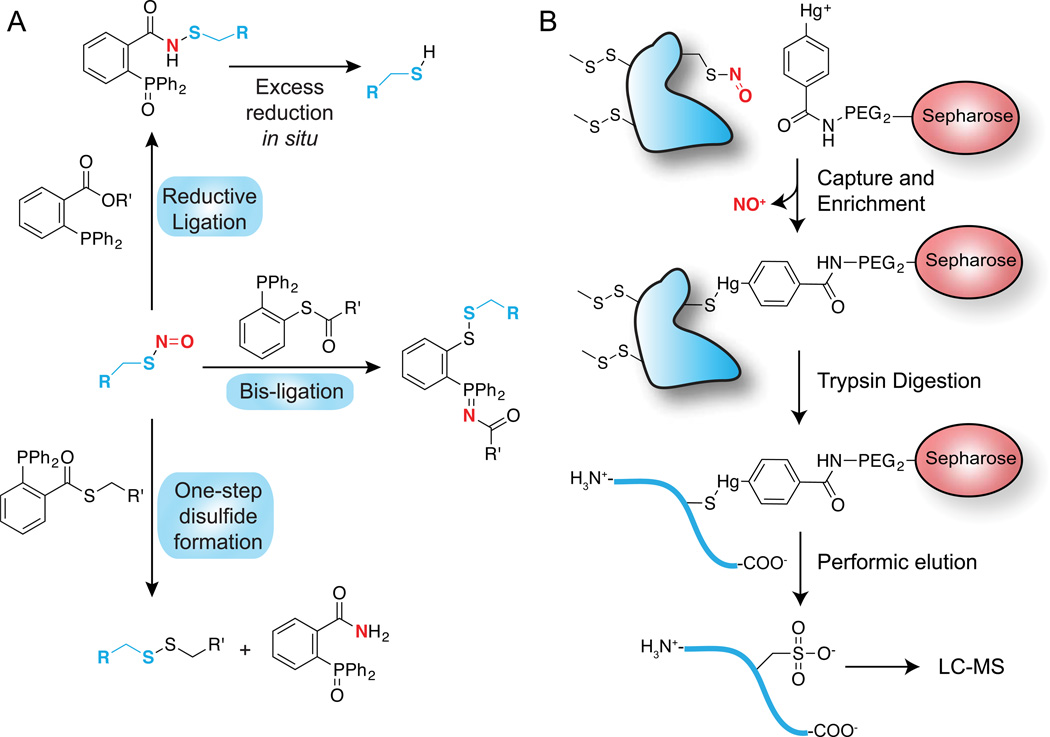
Figure 3.
Chemoselective nitrosothiol labeling methods. In both approaches, free thiols are first blocked by addition of MMTS. (A) Triaryl-phosphine ligation methods. Three reactions are shown that describe recent reports of nitrosothiol-selective phosphine reactions. The reductive ligation approach was demonstrated on fixed cells, but led to over-reduction to the free thiol. The one-step disulfide formation reaction was demonstrated on cell lysates after nitric oxide donor treatment. (B) Phenyl-mercury enrichment of nitrosothiols for proteomic annotation. Sepharose beads or biotin are linked to phenyl-mercury for nitrosothiol enrichment, followed by trypsin digestion. Nitrosylated peptides are released from the resin by perfomic acid oxidation to the sulfonic acid for mass spectrometry annotation.