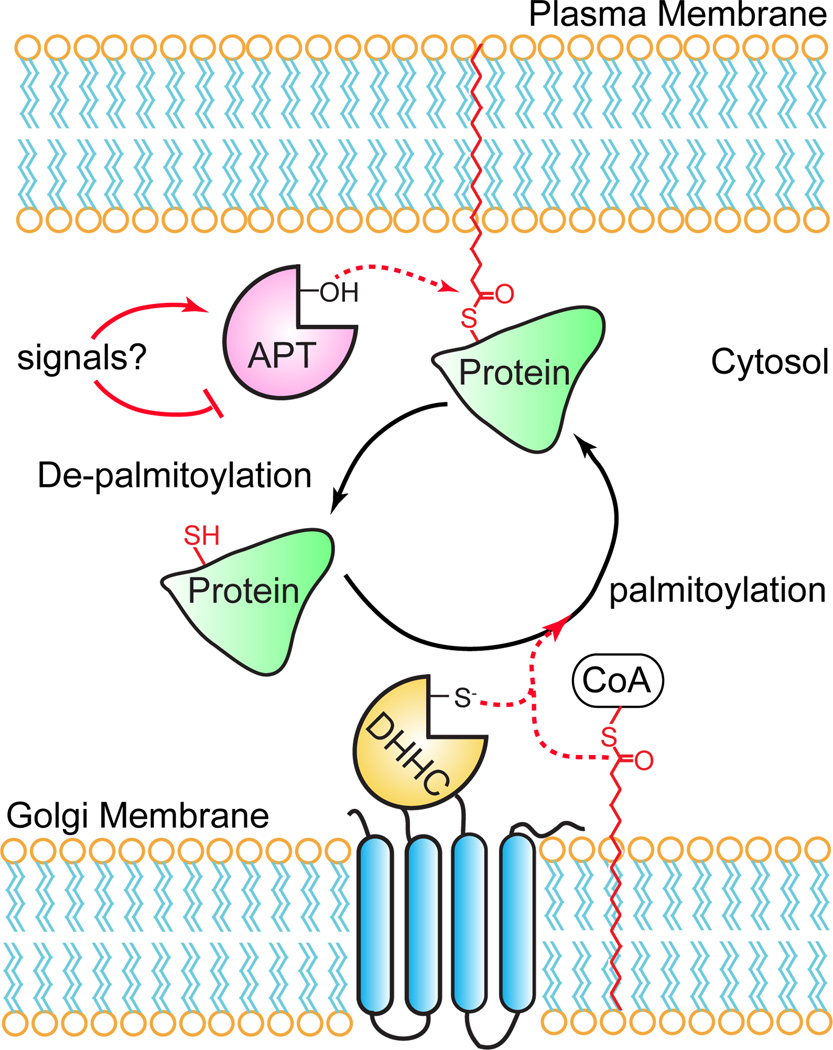
Figure 1. A dynamic S-palmitoylation cycle regulates the membrane association and activity of certain palmitoylated proteins.
Certain stimuli cause an acyl protein thioesterase to catalyze thioester hydrolysis of palmitoylated cysteine residues. De-palmitoylation liberates the protein from the plasma membrane, allowing it to segregate to the cytosol disrupting interactions with membrane-associated signaling complexes. The protein can then undergo re-palmitoylation by Golgi-resident DHHC palmitoyl transferases, transport back to the plasma membrane, and re-associate with signaling partners.