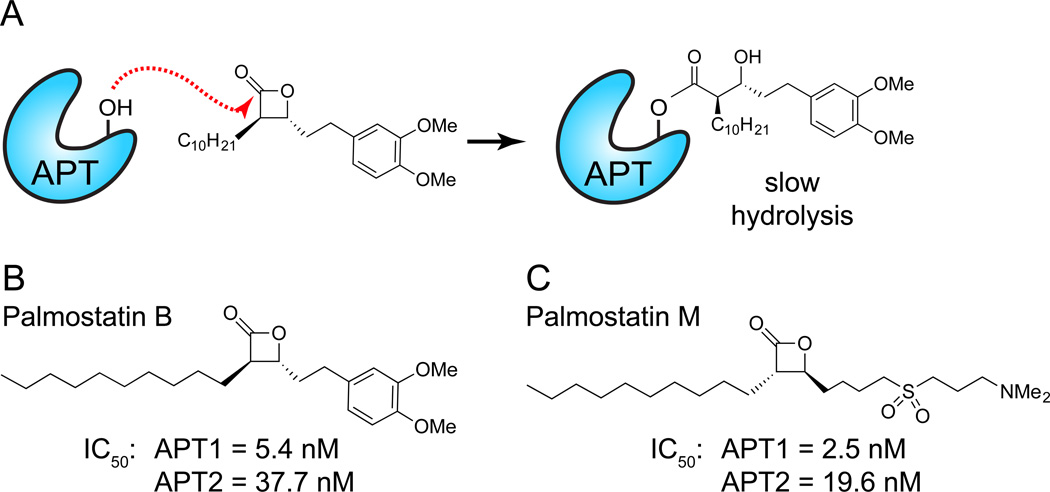
Figure 4. Mechanism-based β-lactones inhibit APT enzymes.
(A) APT competitive enzyme inhibition by Palmostatin B. Attack of the electrophilic lactone by the catalytic serine hydroxyl leads to a transient acyl-enzyme adduct, which slowly hydrolyzes to regenerate the active enzyme. (B) Structure of Palmostatin B. This inhibitor was discovered through the synthesis and screening of a small library of compounds on the basis of active-site similarity between APT1 and gastric lipase, and the fact that β-lactones are known lipase acylating reagents. (C) Structure of Palmostatin M. This inhibitor was developed based on the structural similarity of lysophosphatidyl choline. A common recognition motif was identified, consisting of an electropositive dimethylamino tail imitating lysine and choline, an electronegative sulfoxide to mirror the phosphate, a central electrophilic trans-β-lactone and a lipophilic tail resembling the palmitate moiety to create affinity to the lipid-binding pocket of the enzyme.