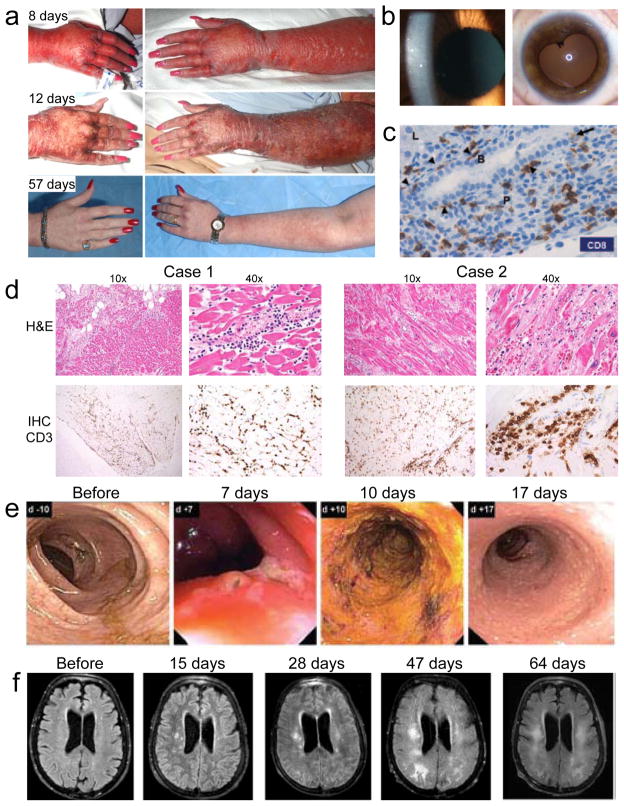
Figure 1.
Autoimmune adverse events in ACT clinical trials. (a) Skin rash at various time points after treatment of a melanoma patient with T cells engineered to express a TCR with high affinity for MART1. (b) Cellular anterior chamber infiltrate in a melanoma patient two weeks after treatment with T cells engineered to express a TCR with high affinity for MART1 (left). Aymptomatic posterior synechiae six months after treatment of the same patient (right)10. (c) Immunohistochemical (IHC) analysis of CD8 expression in a liver biopsy obtained four days after treatment of a renal cell carcinoma patient with T cells transduced with a CAR specific for carbonic anhydrase IX. CD8 T cells line the basal side of (arrowheads) and infiltrate (arrow) the bile duct epithelium. Liver parenchyma (L), portal triangle (P), and bile duct (B) are labeled22. (d) Colonoscopy images from a colon cancer patient at various time points after administration of T cells engineered to express a TCR specific for carcinoembryonic antigen (CEA). Transient severe colitis is evident11. (e) Magnetic resonance imaging of the brain of a melanoma patient after various time points after injection of T cells expressing a receptor that recognizes MAGE-A3 but is cross-reactive with MAGE-A1212. Images show progressive white matter changes consistent with leukomalacia.