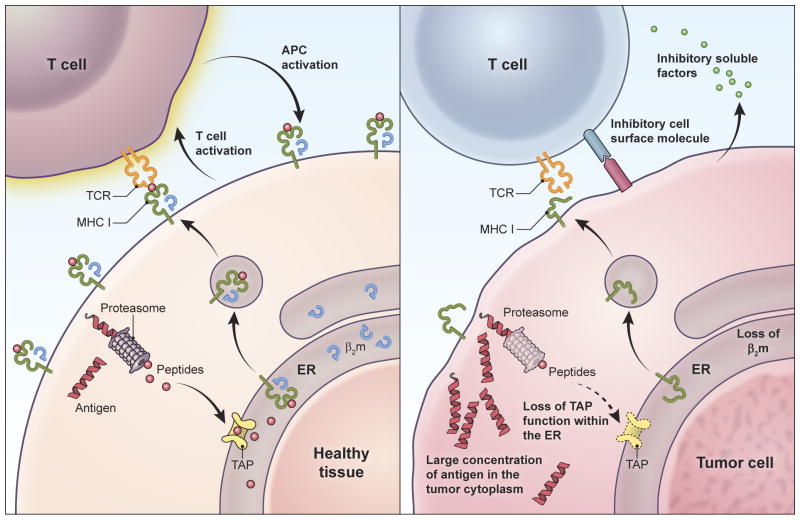
Figure 4.
T cells may target healthy tissues more efficiently than tumors, independent of the relative abundance of target antigen on each tissue. This phenomenon may preclude identification of a therapeutic window based on enhanced sensitivity of tumor compared to healthy tissue to T cell-mediated killing. Because high-avidity T cells have exquisite sensitivity for their target antigens and can recognize even single complexes of target peptide-MHC122, healthy tissues expressing even small quantities of antigen but possessing intact antigen processing and presentation machinery might be highly vulnerable to T cell-mediated killing (left panel). Once initiated, such T cell killing can be amplified by the proliferation of T cells and by the mutual activation of T cells and antigen presenting cells. Despite expressing potentially large quantities of target antigen, tumors are prone to genomic instability, defects in antigen processing and presentation (e.g. defects in transporter associated with antigen processing function (TAP), proteosomal subunits, β-2 microglobulin, and MHC molecules) (right panel). As such they may not present target antigen to engineered T cells. Tumors may be further protected by their production of cell surface and soluble molecules that inhibit T cell activation.