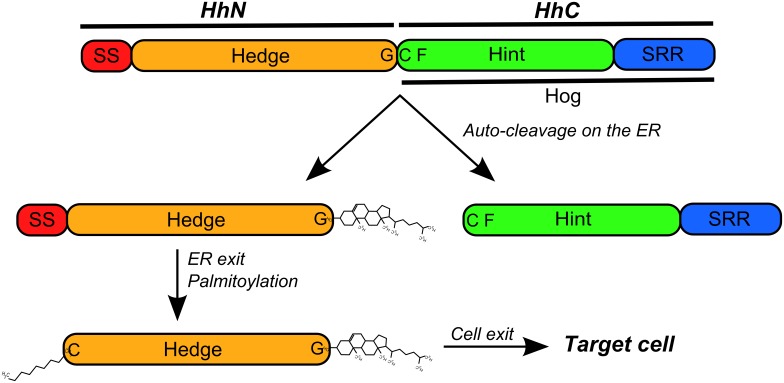
Figure 1. Structural features of the Hh proteins.
The hedgehog proteins are composed by two main domains: the Hedge (N-terminal) and Hog (C-terminal) domains. The Hedge domain forms the HhN portion of the Hh proteins (together with the signaling sequence, SS) that is separated from the rest of protein by an auto-cleavage reaction preformed by the Hog domain [4]. The Hog domain forms the HhC portion of the Hh proteins and shares similarity with self-splicing Inteins on the Hint module [22]. The auto-cleavage reaction occurs on a GCF (glycine-cysteine-phenylalanine) motif that forms the boundary between the two main parts of the Hh proteins, with the cysteine residue initiating a nucleophilic attack on the carbonyl carbon of the preceding residue, the glycine [5]. The sterol-recognition region (SRR) forms the C-terminal region of the Hog domain and binds a cholesterol moiety that acts as an electron donor on a second nucleophilic attack that results in the cleavage of the bound between the glycine and cystein residues and in the attachment of the cholesterol moiety to the glycine residue [5]. After auto-cleavage, the sterified HhN fragment is further palmitoylated on the cystein residue immediately after the SS region [13] and leaves the endoplasmic reticulum (ER) for later export [11].