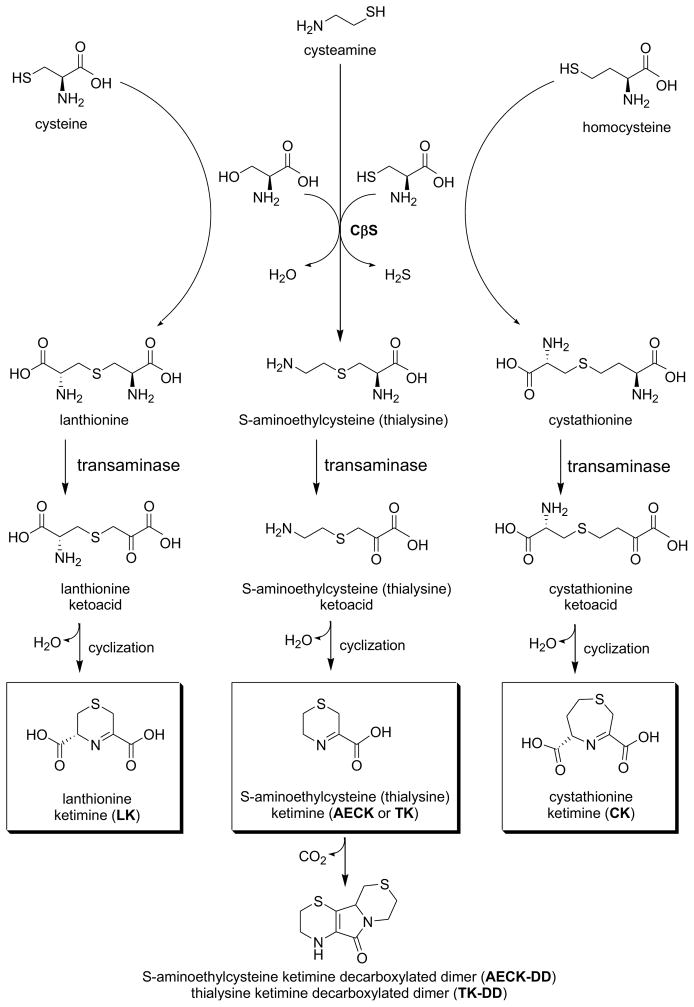
Figure 2. Formation of cyclic thioether ketimines from cystathionine and alternative transsulfuration products.
Lanthionine, thialysine and cystathionine formed via CβS undergo transamination to yield intermediate linear ketoacid amines. Cyclization of these species yields a family of cyclic thioether ketimines. Both the ketimines and decarboxylated dimers formed from their bimolecular oxidative breakdown are present in tissue and have appreciative antioxidant potential in vitro. Cell penetrating derivatives of lanthionine ketimine are becoming appreciated for their antioxidant, neuroprotective and neurotrophic properties and therapeutic potential. Note that the ketimines depicted here exist as tautomers with their corresponding enamines.