Abstract
Background
Children with cancer and hematopoietic stem cell transplant (HSCT) recipients are at high risk for common viral infections. We sought to define the viral etiology of acute respiratory infections (ARI) and identify risk factors.
Methods
Nasal wash samples were collected from pediatric hematology-oncology patients and HSCT recipients with ARI during the 2003–2005 winter seasons. Real-time RT-PCR was performed to detect influenza A (Flu A), influenza B, respiratory syncytial virus (RSV), parainfluenzaviruses (PIV) 1–3, human metapneumovirus (MPV), and human rhinoviruses (HRV). HRV specimens were sequenced and genotyped.
Results
Seventy-eight samples from 62 children were included. Viruses were detected in 31 of 78 samples (40%). HRV were detected most frequently, in 16 (52%) including 5 HRV type C (HRVC); followed by 7 (22%) RSV, 5 (16%) Flu A, 4 (13%) MPV and 2 (6%) PIV2. There was a trend toward higher risk of viral infection for children in daycare. Only 8% of the study children had received infuenza vaccine.
Conclusions
HRV, including the recently discovered HRVC, are an important cause of infection in pediatric oncology and HSCT patients. Molecular testing is superior to conventional methods and should be standard of care, since HRV are not detected by conventional methods.
Keywords: cancer, pediatric, respiratory viruses, real-time RT-PCR, human rhinovirus
INTRODUCTION
Acute respiratory infections (ARI) in childhood cause significant morbidity and mortality (1). Compared to the immunocompetent host, children undergoing myelosuppressive therapy, as well as those who receive hematopoieitic stem cell transplantation (HSCT), are at higher risk for severe disease with common viral infections due to immune compromise (2–10). Respiratory syncytial virus (RSV) is a leading cause of ARI; other major viruses are influenza, human metapneumovirus (MPV), human rhinoviruses (HRV), and parainfluenzaviruses (PIV) (11–16). Recent studies have identified a new species of HRV called HRVC (17–20). These novel HRV are associated with wheezing and asthma (15, 21), but their role in immuncompromised children is not clear. We sought to define viral etiology, including HRV, and identify risk factors for respiratory virus infection among a pediatric hematology-oncology and HSCT patient population and describe associated disease manifestations.
MATERIALS AND METHODS
Study design
This study is based on convenience samples collected from the population of interest. Recruitment began in December 2003 and subjects were enrolled during two winter seasons (between December and March) until December 2005. Children evaluated as inpatients and outpatients in the Hematology-Oncology department for any underlying hematology-oncology problem and/or HSCT who presented with acute respiratory symptoms were eligible. Nasal washes were collected from children with either ≥1 upper respiratory symptom accompanied by a temperature >38.4°C, or ≥1 lower respiratory sign or symptom with or without fever. During sick visits, subjects’ signs and symptoms were recorded on a standardized clinical form that documented the following symptoms: cough, wheezing, fever, rhinorrhea, nasal congestion, sore throat, red eyes, dyspnea, vomiting, diarrhea, hoarseness and myalgia. Subjects or parents were asked about sick contacts at home and daycare or school attendance.
Additional data were collected in a retrospective review of each subject’s electronic medical record including: gender, age, race, ethnicity, oxygen requirement, diagnosis of URI or LRI, radiologic studies, HSCT information (date, autologous or allogeneic, stem cell source), absolute neutrophil and lymphocyte count, immunosuppressive therapy, antiviral prophylaxis, influenza vaccination, antibiotics (prophylactic, new prescription after sick visit), steroid treatment, the presence of graft versus host disease (GvHD), and last chemotherapy. The study was approved by the Vanderbilt Institutional Review Board (IRB).
Criteria for analysis and definitions
Subjects with both a sample and a completed clinical form were included in the analysis. Multiple samples were accepted from subjects with repeated illnesses when separate illness episodes occurred at least one month apart. An infection was defined as the presence of a detectable study virus in the participant’s sample. URI was defined as a positive RT-PCR plus ≥1 observed symptoms including cough, fever (>38.4 °C), rhinorrhea, nasal congestion, hoarseness, red eyes, or pharyngitis. LRI was defined as a positive RT-PCR plus ≥1 observed wheezing, stridor, rales, or new chest radiograph infiltrates, or clinical diagnoses of bronchiolitis, croup, or pneumonia.
Virologic methods
Nasal washes were collected from each subject by instilling sterile saline solution (5mL/nostril for children and 10 mL/nostril for teenagers-young adults) and collecting the effluent in a sterile cup. Samples were placed on ice and processed in the laboratory within 4 hours; each original sample was aliquoted and stored at −80°C. RNA was extracted on an automated instrument (MagMAX-96, ABI) using the MagMAX-96 Viral RNA Isolation Kit (ABI). Real-time RT-PCR assays were performed for individual viruses separately using primers and dual-labeled probes for RSV, influenza A, influenza B, PIV1, PIV2, PIV3, HRV and MPV as previously described (15, 22–26). Twenty-five-μL reaction mixtures containing 5 μL of specimen RNA were tested using the AgPath-ID RT-PCR kit (ABI) on the Step One Plus instrument (ABI). Positive and negative controls were used with each run. All RT-PCR assays were optimized and characterized using RNA runoff transcripts and were capable of detecting <50 RNA transcript copies/reaction (data not shown). All samples were tested by RT-PCR assay for human beta-actin (ABI) to ensure RNA integrity.
Rhinovirus sequencing
Conventional RT-PCR was performed on HRV-positive specimens using primers that amplify a ~600-bp fragment encompassing the VP4/VP2 region (15, 27). Amplified fragments were sequenced bidirectionally on a 3730xl DNA Analyzer (ABI). Sequences were edited and aligned with published HRVA, HRVB, and HRVC (HRV-1B, HRV14, and HRV-QPM) sequences obtained from GenBank using MacVector version 11.0 (MacVector) and phylogenetic analyses performed using MEGA 4.0.(28, 29)
Statistical Analysis
Groups were compared on the continuous variables using Wilcoxon rank sum test and categorical variables using Pearson’s Chi-square test. Data were analyzed using R version 2.10.1. Due to the small number, multivariable analysis was not performed.
RESULTS
Study Population
During the study period 82 samples were collected from 62 subjects evaluated as inpatients or outpatients at the Monroe Carell Jr. Children’s Hospital at Vanderbilt. Four samples were excluded because complete patient information was unavailable; the analysis was based on 78 samples collected during ARI episodes from 62 subjects. Fifty out of 78 subjects provided at least one sample and 12 had ≥2 obtained.
Subject demographic and clinical characteristics are listed in Table 1. The mean age of the 62 participants was 9 years and 60% were male, with 40% of samples from HSCT recipients. The non-HSCT subjects had the following underlying conditions: acute leukemia (n=21), aplastic anemia (n=2), Langerhans cell histiocytosis (n=3), solid tumor (n=5), sickle cell anemia (n=3), myelodysplastic syndrome (n=1), autoimmune neutropenia (n=1) and Hodgkins lymphoma (n=1).
Table 1.
Demographic characteristics of study subjects (N=62).
| Characteristics | N (%) |
|---|---|
| Median age in years (range) | 6.9 (1–23) |
| Gender | |
| Male | 37 (60) |
| Female | 25 (40) |
| Transplant status | |
| HSCT | 25 (40) |
| No transplant | 37 (60) |
| Total samples per participant | |
| 1 sample | 50 (81) |
| 2 samples | 9 (14) |
| 3 samples | 2 (3) |
| 4 samples | 1 (2) |
HSCT: Hematopoietic stem cell transplant
Respiratory viruses detected
At least one respiratory virus was detected by RT-PCR in 31 (40%) of 78 nasal wash samples; these were from 31 distinct subjects. Two different respiratory viruses were detected simultaneously in 3 samples. The viruses detected and the sites of infection are listed in Table 2. HRV were the most frequently detected viruses and were identified in 15 (48%) positive samples; followed by 7 RSV (22%), 5 influenza A (16%), 4 MPV (13%) and 2 PIV2 (6%); influenza B, PIV1 and PIV3 were not detected in any sample.
Table 2.
Respiratory viruses detected and type of respiratory tract infection
| Virus | RT-PCR positive samples N=31 |
LRI N=11 |
URI N=20 |
|---|---|---|---|
| HRV | 15 | 5 | 10 |
| RSV | 4 | 2 | 2 |
| Flu A | 5 | 1 | 4 |
| MPV | 2 | 2 | 0 |
| PIV2 | 2 | 0 | 2 |
| RSV and MPV | 2 | 1 | 1 |
| HRV and RSV | 1 | 0 | 1 |
Human rhinovirus (HRV); Respiratory syncytial virus (RSV); influenza A, (Flu A); human metapneumovirus (MPV); parainfluenza 2 (PIV2)
Five (8%) of the 62 participants had documented history in their EMR of receiving influenza vaccine. Of those 5 participants, one tested influenza A positive by RT-PCR.
HRV species
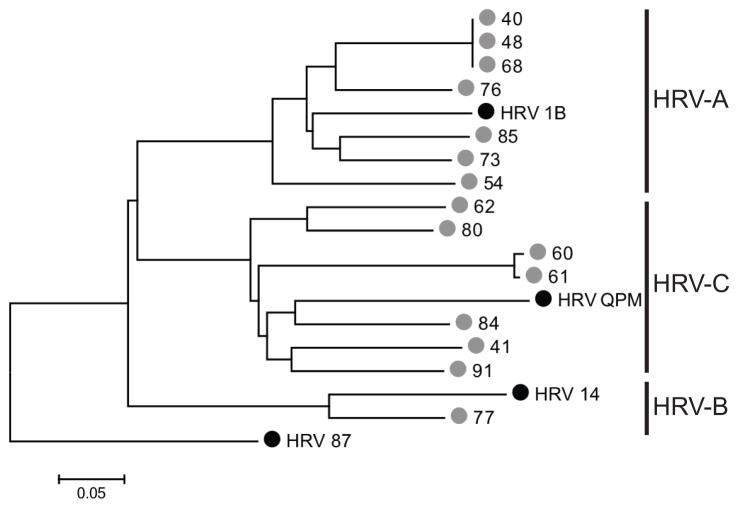
The VP4/VP2 sequence regions correlate with the serotype classification of HRV (15, 20, 27). HRV-positive samples were sequenced using this region: 5 (33%) of those sequenced were HRVC, 9 (60%) HRVA and 1 (7%) HRVB, with substantial genetic diversity between strains (Figure 1). HRV87 was used as an outgroup for phylogenetic analysis (30). Four distinct HRVC genotypes were identified, even among this closed population (Figure 1). The HRVC viruses were more genetically diverse than HRVA viruses. Of the 9 HRVA, 6 were URI and 3 LRI, HRVC were from 3 URI and 2 LRI, and the single HRVB was from URI. Of the five HRV-positive specimens associated with LRI, 3 were from subjects diagnosed with pneumonia by chest radiograph, with no bacterial pathogen identified, and 2 of those 3 were identified as HRVC.
Figure 1.
Phylogenetic tree depicting the relationship of HRV sequences identified in this study. Phylogeny was inferred using Neighbor-Joining as described in Methods. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Black circles indicate prototype GenBank strains; gray circles indicate strains sequenced in this study. Scale bar indicates nucleotide substitutions/site.
Clinical manifestations and factors associated with virus detection
Seventy-two percent of samples were collected from outpatients. Of 31 samples with a virus detected, 64% were from participants with URI and 36% with LRI. The remaining 47 samples were collected from patients who presented with URI or LRI symptoms but without a detectable virus (details shown in Table 2). Cough, fever, rhinorrhea, and nasal congestion were the most prominent presenting symptoms among virus-positive and virus-negative subjects, with no significant differences between groups (not shown). Nine of the 78 ARI episodes were diagnosed as clinical pneumonia by chest radiograph; of those, 4 samples were positive for respiratory viruses (3 HRV positive and 1 MPV). Three of these 4 were treated with antibiotics. None of the patients with respiratory viruses detected required ICU admission and none died.
There was not a statistically significant difference for risk of virus detection between a virus-positive or -negative sample compared for most variables analyzed; lymphocyte count was slightly higher in subjects with virus detected, though there was substantial overlap in the interquartile ranges (Table 3). Analysis of subjects with upper vs. lower respiratory infeciton did not identify any clinical or demographic factors significantly associated with lower respiratory infection (Table 4).
Table 3.
Subject characteristics by virus-positive and virus-negative status.
| Virus-positive N = 31 |
Virus-negative N = 47 |
P value* | 95% Confidence Interval of the difference in % | |
|---|---|---|---|---|
|
| ||||
| CATEGORICAL | No. (%) | No. (%) | ||
| Clinical features | ||||
| Transplant | 15 (48) | 28 (60) | 0.331 | −11.3, 33.7 |
| GvHD (among transplant recipients) | 27 (87) | 44 (94) | 0.321 | −7.2, 20.2 |
| Medication (%) | ||||
| Prophylactic antibiotic | 7 (23) | 4 (9) | 0.091 | −30.7, 2.9 |
| New antibiotic prescribed | 8 (27) | 5 (11) | 0.071 | −33.7, 2.1 |
| Antiviral prophylaxis | 16 (52) | 25 (54) | 0.811 | −19.9, 25.4 |
| Immunosuppressive | 19 (61) | 30 (65) | 0.721 | −18.0, 25.8 |
| Steroids | 16 (52) | 27 (59) | 0.541 | −15.4, 29.6 |
| Social features (%) | ||||
| Daycare/school attendance | 18 (60) | 34 (81) | 0.051 | 0.4, 41.5 |
| Sick contacts | 11 (48) | 24 (67) | 0.151 | −3.3, 41.0 |
| CONTINUOUS | Median (lower, upper quartile) | Median (lower, upper quartile) | ||
| Absolute neutrophil count (per mm3) | 3.55 (2.50, 5.80) | 2.30 (0.98, 4.95) | 0.072 | |
| Absolute lymphocyte count (per mm3) | 1.35 (0.60, 2.15) | 0.60 (0.30, 1.17) | 0.012 | |
| Time since last chemotherapy (days) | 142 (2, 453) | 14 (2, 76) | 0.072 | |
GvHD: graft versus host disease.
P value for univariable analysis; due to the small number, multivariable analysis was not performed. Tests used:
Pearson test;
Wilcoxon test
Table 4.
Subject characteristics by upper vs. lower respiratory infection status.
| Upper Respiratory Infection N = 48 |
Lower Respiratory Infection N = 21 |
P value | 95% Confidence Interval of the difference in % | |
|---|---|---|---|---|
|
| ||||
| CATEGORICAL | No. (%) | No. (%) | ||
| Clinical features | ||||
| Transplant | 27 (56) | 13 (62) | 0.661 | −19.4, 30.7 |
| GvHD (among transplant recipients) | 42 (88) | 20 (95) | 0.331 | −5.3, 20.8 |
| Medication (%) | ||||
| Prophylactic antibiotic | 6 (13) | 4 (19) | 0.501 | −13.0, 25.6 |
| New antibiotic prescribed | 7 (15) | 5 (25) | 0.321 | −11.0, 31.2 |
| Antiviral | 25 (53) | 13 (62) | 0.501 | −16.4, 33.8 |
| Immunosuppressive | 28 (60) | 15 (71) | 0.351 | −11.9, 35.6 |
| Steroids | 28 (60) | 9 (43) | 0.201 | −42.0, 8.6 |
| Social features (%) | ||||
| Daycare/school attendance | 35 (74) | 12 (60) | 0.241 | −38.8, 9.8 |
| Sick contacts | 25 (62) | 10 (56) | 0.621 | −32.2, 18.3 |
| CONTINUOUS | Median (lower, upper quartile) | Median (lower, upper quartile) | ||
| Absolute neutrophil count (per mm3) | 3.20 (1.30, 4.95) | 3.00 (1.50, 7.30) | 0.302 | |
| Absolute lymphocyte count (per mm3) | 0.90 (0.42, 1.58) | 0.70 (0.30, 2.50) | 0.772 | |
| Time since last chemotherapy (days) | 26 (2, 211) | 10 (2, 248) | 0.642 | |
Some subjects did not have upper vs. lower respiratory infection documented in their case report and thus were excluded from this analysis.
P value for univariable analysis; due to the small number, multivariable analysis was not performed. GvHD: graft versus host disease. Tests used:
Pearson test;
Wilcoxon test
One participant with an HRV-positive specimen was subsequently found on biopsy to have a Mycobacterium avium complex lung nodule. Three samples from symptomatic children had other possible pathogens in addition to the respiratory viruses studied; one patient with presumed Enterococcus sp. pneumonia diagnosed by bronchoalveolar lavage culture, one with a Candida albicans sputum culture; and a third with herpes simplex pneumonitis diagnosed by DFA and culture of bronchoalveolar lavage. No other bacterial, fungal or viral co-infections were reported in participants’ medical records.
Comparison of research with clinical testing
Forty-three of the 78 specimens were submitted to the clinical laboratory for rapid antigen testing: 33 for Flu A/B and RSV; 5 for Flu A/B; and 5 for RSV alone. Of these, 18 were RT-PCR positive but only one was Flu A positive by rapid antigen test. DFA performed in the hospital laboratory detected one case of influenza A, 1 case of RSV and 1 case of PIV-2; the same viruses were detected by RT-PCR. Thus, routine clinical testing for viral infection yielded only 4 (9%) confirmed viral cases among the 43 subjects tested. Thirty-five of 78 were not tested by the treating physician, and 13 of these were positive for a virus by RT-PCR.
DISCUSSION
In this study, RT-PCR was used to detect respiratory viruses in nasal washes from immunocompromised children with ARI symptoms. We identified respiratory viruses in 31 of 78 (40%) nasal wash samples by RT-PCR, in contrast with only 4 detected by routine clinical testing. This emphasizes the importance of using sensitive molecular techniques to detect viral etiologic agents in this high-risk population. In addition, real-time RT-PCR permitted detection of some viruses rarely identified by conventional methods, such as HRV and MPV. National proficiency testing surveys performed by the College of American Pathologists in 2013 suggests that ~200 hospital laboratories use molecular methods for respiratory virus testing (unpublished data; www.cap.org). Of these laboratories, slightly more than half use multiplex assays, while the rest use limited assays that do not detect HRV or MPV. The most common viruses we identified were HRV in nearly half of virus-positive episodes, with one-third of these HRVC.
Other studies of respiratory virus infections have been conducted in oncology and HSCT patients, including children (2, 6–9, 31, 32). However, many studies use conventional methods or focus on detection of a single virus. A retrospective Korean study reported PIV as the most common virus, followed by RSV (33); however, that study used conventional methods including culture and DFA, which rarely detect HRV and MPV (15, 24). The study only identified a virus in 6% of patients, likely due to insensitive methods, and no HRV were detected. A retrospective study in Boston included conventional testing, and PCR only if ordered by the clinician; the study reported only RSV, adenovirus, influenza, and PIV, and included only virus-positive episodes, so incidence was not calculated (34). One prospective study compared multiplex PCR with conventional testing in a pediatric population; interestingly, that study also found HRV to be the most common virus at 63% (35). That study did not genotype or speciate HRV specimens. As in our study, HRV infection was associated with LRI as well as URI. Only one report included genotyping of HRV in HSCT recipients, and that study included only adults; HRV was detected in 8% of 229 episodes of ARI, and 39% of virus-positive illnesses; our study identified HRV in 19% of all illnesses, and 48% of the virus-positive specimens. In this Australian cohort, HRVC was the most common species and was associated with both URI and LRI (36).
HRV are capable of causing clinical symptoms and pneumonia in adult patients with hematologic malignancies (37). In addition, HRVA, HRVB, and HRVC can cause URI and LRI and are associated with wheezing, otitis media and sinusitis (19, 20). The recently discovered HRVC have been linked with acute wheezing and asthma (15, 21). Some reports suggest that HRVC are more virulent and exhibit different demographic and epidemiologic characteristics compared to HRV A (15, 21, 38). Our data suggest that HRV-C are important pathogens in immunocompromised children.
Several factors are reported to increase the risk for severity of viral infection in children with hematologic disorders, cancer and HSCT recipients, including lymphopenia, steroid use, and GvHD (39, 40). In our study, none of the measured factors was statistically significantly associated with respiratory viral infection; of note, the detection of a virus in the subjects may have reflected medically significant respiratory disease leading to testing rather than differences in exposure to viruses. In addition, subjects without a virus detected may have had heterogeneous etiologies (e.g. allergic, bacterial, or fungal). The inability to identify significant associations may be due to the small number of subjects; alternatively, this entire population may be at risk for medically significant respiratory virus infections. In support of this, other similar studies failed to identify clinical and demographic risk factors except for the presence of LRI at presentation (33, 34, 41).
A minority of subjects had received inactivated influenza vaccine, yet influenza was identified in five of the children. Others have identified influenza as an important pathogen in immunocompromised children (42, 43). While antibody responses to influenza vaccine may be lower among immunocompromised children (44, 45), inactivated influenza vaccine is safe and recommended annually for high-risk persons. Others have found that the likelihood of receiving influenza vaccination was only slightly higher in immunocompromised children, even in a large HMO (46). Our data suggest that influenza vaccine recommendations may not be routinely followed in all centers.
Our study has several limitations. First, due to the small sample size, the collective analysis of the risk factors for respiratory viral infection may be inadequately powered. We detected relatively few infections with each individual virus. We did not test for all known respiratory viruses; however, we did test for the eight most common pediatric respiratory viruses using sensitive individual real-time RT-PCR. We did not include asymptomatic controls; literature is conflicting on how common asymptomatic infection is in the HSCT population (9, 40, 41). It is possible that the HRV we detected was not the causative pathogen of the clinical illness; however, we excluded other viruses by RT-PCR. Thus, we think it likely that the HRV we detected were truly associated with disease.
In summary, our findings suggest that HRV, including the recently discovered species HRVC, are important pathogens in the pediatric HSCT population. Notably, conventional methods and some molecular assays will fail to detect these viruses. Molecular testing for multiple viruses should be considered the standard of care for immunocompromised patients with ARI requiring hospitalization.
Article summary.
HRVC is a common cause of acute respiratory infections in pediatric oncology patients and stem cell transplant recipients.
Footnotes
Author contributions: Performed experiments: CL, JAD, EKM, JVW. Recruited subjects: JAD, HF. Statistical analysis: MX, BRS. Funding secured by: CL, EH, JAD, JVW. Conceived project, wrote manuscript: CL, JAD, NBH, EH, EKM, BRS, HF, JVW. JAD was supported by NIH/ HD043483. CL and EH were supported by NIH/FIC TW007697. JVW was supported by NIH/NIAID R01 AI085062.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Bredius RG, Templeton KE, Scheltinga SA, Claas EC, Kroes AC, Vossen JM. Prospective study of respiratory viral infections in pediatric hemopoietic stem cell transplantation patients. Pediatr Infect Dis J. 2004;23:518–522. doi: 10.1097/01.inf.0000125161.33843.bb. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Powell KR, MacDonald NE, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 4.Lazar I, Canaan A, Weibel C, Kahn JS. Novel mutations in the respiratory syncytial virus G gene identified in viral isolates from a girl with severe combined immune deficiency treated with intravenous immune globulin. J Clin Virol. 2006;37:168–173. doi: 10.1016/j.jcv.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Moscona A. Management of respiratory syncytial virus infections in the immunocompromised child. Pediatr Infect Dis J. 2000;19:253–254. doi: 10.1097/00006454-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Cost C, Brock E, Adams-Huet B, Siegel JD, Ardura MI. 2009 pandemic influenza A (H1N1) virus infection in pediatric oncology and hematopoietic stem cell transplantation patients. Pediatr Blood Cancer. 2011;56:127–133. doi: 10.1002/pbc.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1520–1527. doi: 10.1016/j.bbmt.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. The Journal of infectious diseases. 2010;201:1404–1413. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood. 2010;115:2088–2094. doi: 10.1182/blood-2009-09-244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JV, Edwards KM, Weinberg GA, et al. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–1898. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. e101. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42:297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EK, Khuri-Bulos N, Williams JV, et al. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J Clin Virol. 2009;46:85–89. doi: 10.1016/j.jcv.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuri-Bulos N, Williams JV, Shehabi AA, et al. Burden of respiratory syncytial virus in hospitalized infants and young children in Amman, Jordan. Scand J Infect Dis. 2010;42:368–374. doi: 10.3109/00365540903496544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali SA, Gern JE, Hartert TV, et al. Real-world comparison of two molecular methods for detection of respiratory viruses. Virol J. 2011;8:332. doi: 10.1186/1743-422X-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klemenc J, Asad Ali S, Johnson M, et al. Real-time reverse transcriptase PCR assay for improved detection of human metapneumovirus. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2012;54:371–375. doi: 10.1016/j.jcv.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. The New England journal of medicine. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27:1923–1927. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savolainen C, Blomqvist S, Mulders MN, Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol. 2002;83:333–340. doi: 10.1099/0022-1317-83-2-333. [DOI] [PubMed] [Google Scholar]
- 28.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2008 doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4. 0. Molecular biology and evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 30.Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4-based molecular diagnosis. Intervirology. 2002;45:136–141. doi: 10.1159/000065866. [DOI] [PubMed] [Google Scholar]
- 31.Christensen MS, Nielsen LP, Hasle H. Few but severe viral infections in children with cancer: a prospective RT-PCR and PCR-based 12-month study. Pediatr Blood Cancer. 2005;45:945–951. doi: 10.1002/pbc.20469. [DOI] [PubMed] [Google Scholar]
- 32.Kuypers J, Campbell AP, Cent A, Corey L, Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009;11:298–303. doi: 10.1111/j.1399-3062.2009.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeng SH, Yoo HS, Choi SH, et al. Impact of parainfluenza virus infection in pediatric cancer patients. Pediatr Blood Cancer. 2012;59:708–710. doi: 10.1002/pbc.23390. [DOI] [PubMed] [Google Scholar]
- 34.Lo MS, Lee GM, Gunawardane N, Burchett SK, Lachenauer CS, Lehmann LE. The impact of RSV, adenovirus, influenza, and parainfluenza infection in pediatric patients receiving stem cell transplant, solid organ transplant, or cancer chemotherapy. Pediatric transplantation. 2013;17:133–143. doi: 10.1111/petr.12022. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan A, Gu Z, Smith T, et al. Prospective detection of respiratory pathogens in symptomatic children with cancer. Pediatr Infect Dis J. 2013;32:e99–e104. doi: 10.1097/INF.0b013e31827bd619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson PE, Gilroy NM, Faux CE, et al. Human rhinovirus C in adult haematopoietic stem cell transplant recipients with respiratory illness. J Clin Virol. 2013;56:255–259. doi: 10.1016/j.jcv.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murali S, Langston AA, Nolte FS, Banks G, Martin R, Caliendo AM. Detection of respiratory viruses with a multiplex polymerase chain reaction assay (MultiCode-PLx Respiratory Virus Panel) in patients with hematologic malignancies. Leuk Lymphoma. 2009;50:619–624. doi: 10.1080/10428190902777665. [DOI] [PubMed] [Google Scholar]
- 38.Linder JE, Kraft DC, Mohamed Y, et al. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131:69–77. e61–66. doi: 10.1016/j.jaci.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–243. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 40.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan A, Flynn P, Gu Z, et al. Detection of respiratory viruses in asymptomatic children undergoing allogeneic hematopoietic cell transplantation. Pediatr Blood Cancer. 2013;60:149–151. doi: 10.1002/pbc.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito S, Cecinati V, Scicchitano B, et al. Impact of influenza-like illness and effectiveness of influenza vaccination in oncohematological children who have completed cancer therapy. Vaccine. 2010;28:1558–1565. doi: 10.1016/j.vaccine.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tasian SK, Park JR, Martin ET, Englund JA. Influenza-associated morbidity in children with cancer. Pediatr Blood Cancer. 2008;50:983–987. doi: 10.1002/pbc.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito S, Cecinati V, Russo FG, Principi N. Influenza vaccination in children with cancer receiving chemotherapy. Hum Vaccin. 2009;5:430–432. doi: 10.4161/hv.5.6.7942. [DOI] [PubMed] [Google Scholar]
- 45.Shahgholi E, Ehsani MA, Salamati P, Maysamie A, Sotoudeh K, Mokhtariazad T. Immunogenicity of trivalent influenza vaccine in children with acute lymphoblastic leukemia during maintenance therapy. Pediatr Blood Cancer. 2010;54:716–720. doi: 10.1002/pbc.22421. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura MM, Lee GM. Influenza vaccination in adolescents with high-risk conditions. Pediatrics. 2008;122:920–928. doi: 10.1542/peds.2007-3032. [DOI] [PubMed] [Google Scholar]