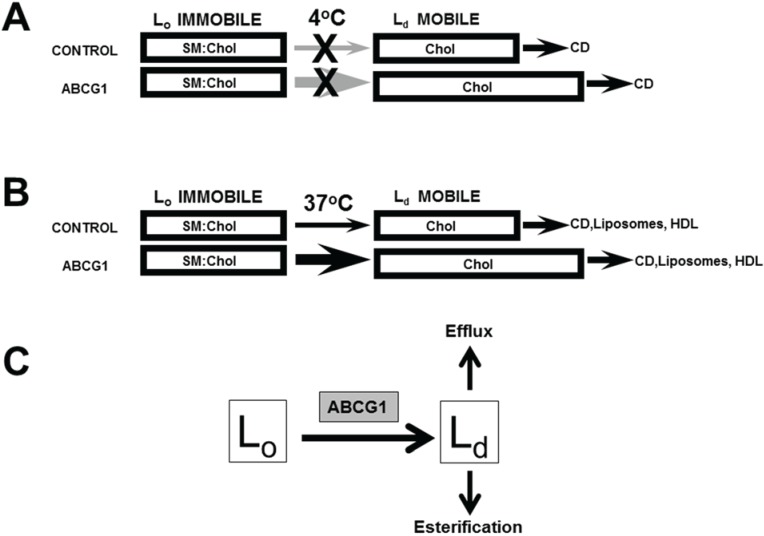
Figure 8.
Model of ABCG1-mediated mobilization of cellular cholesterol for efflux and esterification. ABCG1 increases the flux of PM (A) and cellular (B) cholesterol (FC) between SM-associated FC in Lo membrane domains and non-SM-associated FC in membrane domains Ld pools. (A) At 4 °C, since FC movement from intracellular compartments to the PM as well as movement between Lo and Ld membrane domains at the PM is blocked, CD removes FC only from mobile Ld PM FC pools, which are expanded by ABCG1, as shown. (B) At 37 °C, CD, PC liposomes, and HDL remove mobile FC pools from the PM and intracellular compartments, mostly LE. Depletion of mobile FC pools results in the movement of FC from Lo to Ld membrane domains and subsequent removal by extracellular lipid acceptors from mobile pools. ABCG1 enhances movement of FC between Lo and Ld membrane domains in the PM and LE, thereby increasing FC efflux. (C) ABCG1 residing at the phase boundary between Lo and Ld membrane domains at the PM and LE appears to increase the rate of flux of cholesterol between Lo and Ld membrane domains, and therefore the size of the pool available to desorb to acceptors and in addition, may increase the chemical potential of Ld cholesterol. The mobile pools of FC in Ld membrane domains are available for removal on either side of the membrane lipid bilayer, by extracellular acceptors (efflux), or, by cytosolic carrier proteins, which promote movement to intracellular sites of esterification.