Figure 1.
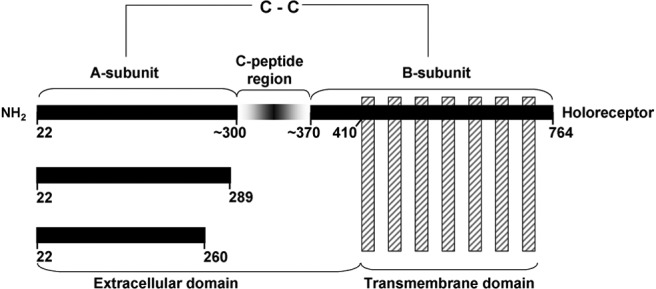
Schematic representation of TSHR components. Intramolecular cleavage of the TSH holoreceptor on the cell surface results in A- and B-subunits linked by disulfide bonds (C-C). This process is associated with deletion of an intervening C-peptide region with indistinct borders, but approximating amino acid residue 300 at the C terminus of the A-subunit and amino acid residue 370 at the N terminus of the B-subunit (10–12). Residue 22 represents the N terminus of the TSHR after signal peptide removal. The purified, recombinant TSHR A-subunit that is the subject of the present report extends to residue 289, the latter chosen because the Arg and Lys cluster in this region was a potential cleavage site and because TSHR-289 was secreted by transfected mammalian cells more effectively than a slightly longer A-subunit. The crystal structure of the A-subunit truncated further upstream at residue 260 has been solved (structure data bank PDB IDs: 3GO4 and 2XWT).
