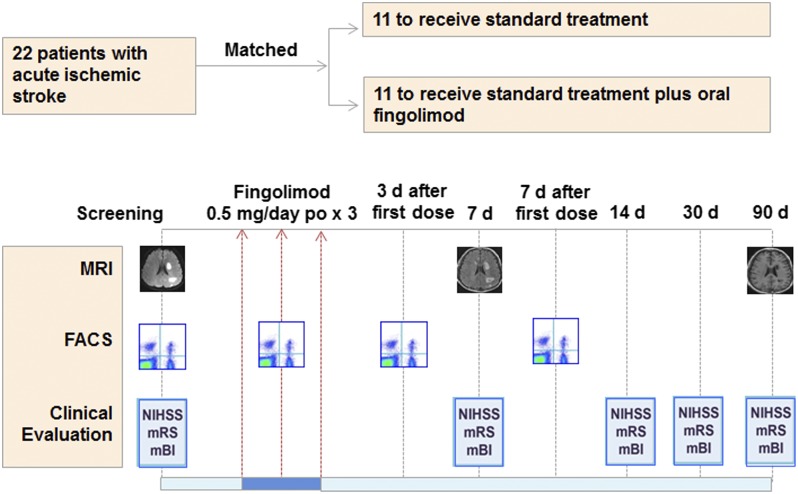
Fig. 1.
Effects of fingolimod in patients with AIS: trial profile. Twenty-two patients with AIS, who exceeded therapeutic window for tPA upon enrollment, were assigned into one of two groups with matched clinical characteristics and subtypes of infarct. All were treated with conventional stroke management and half (n = 11) also received fingolimod (FTY720, Gilenya, Novartis) 0.5 mg orally once daily for 3 consecutive days at the indicated time points. Counts of circulating lymphocyte subsets were monitored by flow cytometry. Clinical assessments (NIHSS, mRS, and mBI) were conducted at the indicated time points. Alterations of infarct volume and microvascular permeability were measured by MRI at the indicated time points.