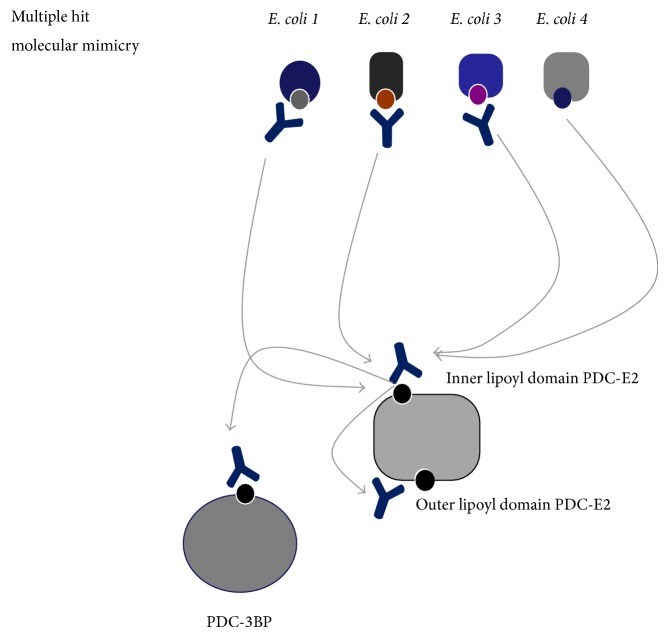
Figure 1.
A microbial/self-multiple hit mechanism of molecular mimicry including several primary biliary cirrhosis- (PBC-) specific autoepitopes and their E. coli mimics (numbered 1–4 corresponding to those with reactivity depicted in Table 2) is likely involved in the induction of antimitochondrial antibody (AMA) responses in PBC. We propose that a multiple hit mechanism of intra- and intermolecular mimicry is operated at the B-cell level. This mechanism involves several mimics from various E. coli proteins which share a high degree of homology with the major mitochondrial autoepitope located at the inner lipoyl domain of the pyruvate dehydrogenase complex E2 subunit (PDC-E2). Urinary tract infections initiate an immune response against the E. coli mimics which in turn cross-react with the human mitochondrial autoantigens (arrows). Autoantibody responses against the human ILD PDC-E2 autoepitope initiate cross-reactive response to the mimicking sequences of the outer lipoyl domain of PDC-E2 and its mimic on the E3 binding protein (E3BP) of PDC (arrows). This multiple hit intra- (between the inner and the outer lipoyl domain of the same protein) and inter- (between different self-proteins and microbial proteins) mechanism of molecular mimicry may explain several specificities of the multiantigen specificities seen in PBC, as well as in other autoimmune diseases.