Figure 1.
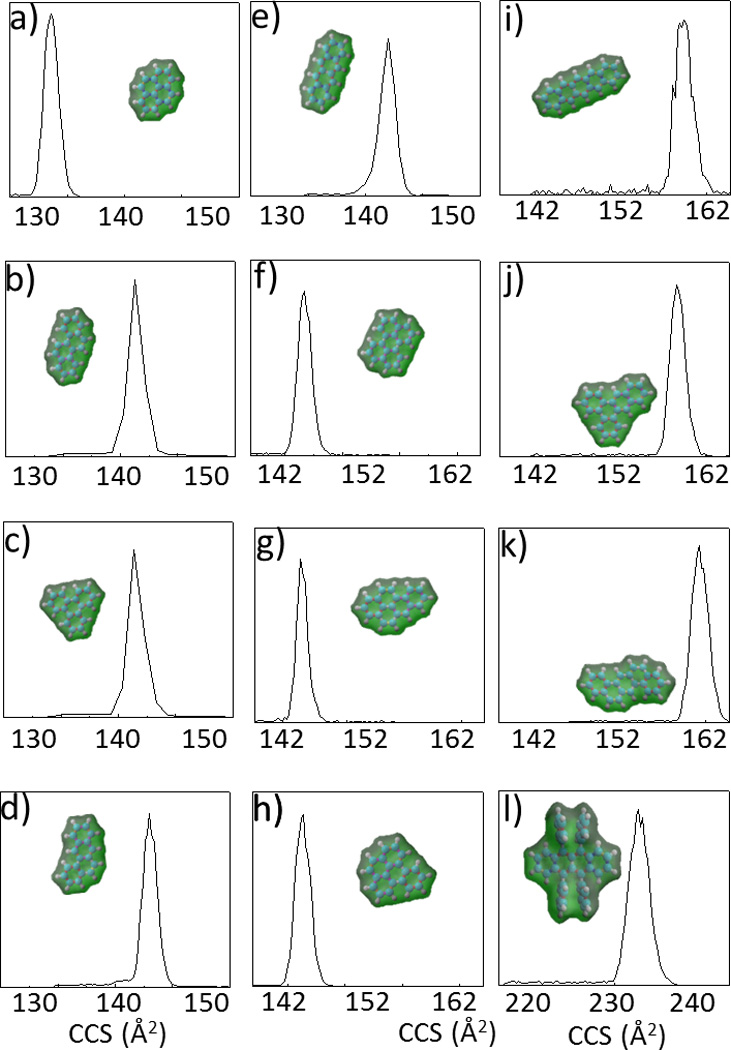
Typical TIMS spectra (Tramp=500ms) of a) Pyrene (R=90), b) Chrysene (R=103), c) Triphenylene (R=107), d) 1,2- Benzanthracene (R=98), e) 2,3- Benzanthracene (R=96), f) Perylene (R=113), g) Benzo(a)pyrene (R=133), h) Benzo(e)pyrene (R=102), i) Pentacene (R=96), j) 1,2,3,4- Dibenzanthracene (R=155), k) 1,2:5,6 Dibenzanthracene (R=98) and l) Rubrene (R=125). In the inset, the DFT/B3LYP/6-31++G(d,p) isosurface and a space-filled model representation of the molecular ions is
