Abstract
The metabotropic glutamate (mGlu) receptors are critically involved in enabling the persistency of forms of synaptic plasticity that are believed to underlie hippocampus-dependent memory. These receptors and in particular, mGlu5, are also required for hippocampus-dependent learning and memory. In the hippocampus, synaptic plasticity is one of the mechanisms by which spatial information may be represented. Another mechanism involves increased firing of place cells. Place cells increase their firing activity when an animal is in a specific spatial location. Inhibition of factors that are essential for synaptic plasticity, such as N-methyl-d-aspartate receptors or protein synthesis, also impair place cell activity. This raises the question as to whether mGlu receptors, that are so important for synaptic plasticity and spatial memory, are also important for place cell encoding. We examined location-dependent place cell firing i.e. place fields. We observed that antagonism of mGlu5, using 2-methyl-6-(phenylethynyl) pyridine (MPEP) had no effect on place field profiles in a familiar environment. However, in a novel environment mGlu5-antagonism affected long-term place field stability, reduced place cell firing and spatial information. These data strongly suggest a role for mGlu5 in the mechanisms underlying informational content and long-term stability of place fields, and add to evidence supporting the importance of these receptors for hippocampal function.
Keywords: place fields, mGlu5, rat
Introduction
Group I metabotropic glutamate (mGlu) receptors that consist of the receptor subtypes 1 (mGlu1) and 5 (mGlu5) are critically involved in persistent hippocampal synaptic plasticity and spatial learning (Balschun and Wetzel, 1998,2002; Balschun et al., 1999,2006; Naie and Manahan-Vaughan, 2004,2005; Manahan-Vaughan and Braunewell, 2005; Bikbaev et al., 2008; Popkirov and Manahan-Vaughan, 2011).
Whereas the activation of N-methyl-d-aspartate (NMDA) receptors is critically required for the induction phase of synaptic plasticity (Dudek and Bear, 1992; Bear and Malenka, 1994; Hrabetova and Sacktor, 1997; Manahan-Vaughan, 1997; Hrabetova et al., 2000; Raymond et al., 2000), the activation of mGlu receptors is crucial for the maintenance phase of plasticity (Manahan-Vaughan, 1997,2000; Bikbaev et al., 2008). Furthermore, induction of both persistent (>24 h) LTP (Manahan-Vaughan et al., 2003), and the acquisition of spatial memories (Riedel et al., 2000) leads to increased hippocampal expression of mGlu5. In addition, dendritic protein synthesis is triggered by mGlu5 (Huber et al., 2000,2001). This suggests that mGlu5 contributes to synaptic restructuring that upholds persistent information storage and bridges the gap between acute NMDA receptor activation and the triggering of downstream cascades necessary for protein synthesis (Mukherjee and Manahan-Vaughan, 2013).
Hippocampal place cells exhibit a spatially restricted firing pattern, and are active only when animals are in a specific location of an environment (O’Keefe and Dostrovsky, 1971). There are two main features of place cells that make them important in the spatial memory system. First, place cells tend to be stable by repeated exposure to the same environment. This kind of stability can last for days, weeks, or even months (Thompson and Best, 1990). Second, when an animal enters a new environment, the firing pattern of place cells changes in an unpredictable manner (known as remapping). Place cells may shift their firing rate, change their place field location or switch on or off. Once place fields are newly formed, they are preserved, and can be stable regardless of their activity or lack of it in another environment (Bostock et al., 1991; Wilson and McNaughton, 1993).
Long-term stability of newly formed place fields is impaired by blocking NMDA receptors (Kentros et al., 1998), blocking protein synthesis in the brain (Agnihotri et al., 2004), or deleting the transcription factor zif268/egr1 (Renaudineau et al., 2009), all of which are essential for long-term synaptic plasticity. On the other hand, afferent stimulation that induces LTP, abolishes existing place fields, creates new place fields, and rearranges the temporal relationship within neighboring place cells (Dragoi et al., 2003), all of which are consistent with “remapping” upon entering a novel environment.
The abovementioned findings show interesting parallels with synaptic plasticity: NMDA receptors are crucial for the induction phase of synaptic plasticity (Dudek and Bear, 1992; Mulkey and Malenka, 1992), and transcription and protein synthesis are necessary in the very late phases (Frey et al., 1988; Manahan-Vaughan et al., 2000). Given the importance of mGlu receptors and in particular mGlu5 for persistent synaptic plasticity, this provokes the question as to whether mGlu5 plays a role in place field formation and/or stability. In this study, we addressed this issue by examining changes in place field stability, firing frequencies and spatial selectivity by blocking mGlu5 during navigation in spatial environments.
Materials and Methods
Subjects
The present study was carried out in accordance with the European Communities Council Directive of September 22, 2010 (2010/63/EU) for care of laboratory animals. All experiments were performed according to the guidelines of the German Animal Protection Law and were approved by the North Rhine-Westphalia State Authority (Bezirksamt, Arnsberg). All efforts were made to reduce the number of animals used.
Male Wistar rats (8- to 9-weeks old) were housed individually and maintained on a 12-h light/12-h dark cycle. The animals were given sufficient food to maintain 90% of their free-feeding weight and ad libitum access to water. They were handled individually for 10 min per day, 1 week before surgery.
Electrodes and Microdrives
One lightweight microdrive (Axona, St. Albans, UK) was chronically implanted in each rat (8–9 weeks at the time of surgery). Each microdrive held four tetrodes made of four twisted bundles of Formvar-coated electrodes (25 µm) platinum-iridium wires (A-M systems, USA). The tetrodes were strengthened respectively with cyanoacrylate glue and inserted into a cannula, which was attached to the microdrive. One full rotation of the mechanical drive produces a vertical movement of 200 µm without rotating the cannula or the electrodes.
Surgery
Each rat was chronically implanted with a microdrive as follows. Animals were anaesthetized with an initial dose of sodium pentobarbital (52 mg kg−1, i.p.) and placed in a stereotactic unit. Body temperature was monitored throughout the operation and the anaesthetic dose was adjusted to maintain surgical anesthesia. A hole was drilled (1.2 mm diameter) over the right hippocampus. The tetrodes were placed in the cortex just above the CA1 hippocampal subfield (bregma −3.8 mm AP, 3.0 mm ML, and 1.5 mm DV). They were lowered into the CA1 cell layer in the screening phase after surgery, by turning the microdrive. So as to protect exposed part of tetrodes between the skull surface and the bottom of the cannula, a sleeve made of 19-gauge tubing was pulled down over the exposed tetrodes to a depth just below the skull surface, the top of which overlapped the cannula. Three holes were drilled in the frontal, parietal and occipital bone respectively, into which small jewelers’ screws were inserted. The microdrive was then anchored to the jewelers’ screws and the skull surface by dental acrylic (Paladur, Heraeus Kulzer GmbH). One of screws also served as the electrical ground. The wound was dusted with chlorhexidine antiseptic powder (Riemser, Germany). The animals were treated before and after surgery with an analgesic (Meloxicam, Vetmedca GmbH, Ingelheim, Germany). The animals were allowed at least 7 days to recover from surgery before screenings were conducted. During this period, they were monitored closely for infection or distress and handled regularly.
Drug Treatment
The negative allosteric mGlu5 modulator 2-methyl-6-(phenylethynyl) pyridine (MPEP; Tocris) was dissolved in 0.9% NaCl. MPEP or vehicle was injected intraperitoneally (i.p.) according to body weight (30 mg kg−1 or an equivalent volume of vehicle: ml kg−1). MPEP or vehicle (0.9% NaCl) was given 30 min prior to the next recording trial to ensure adequate time for the drug to reach the brain and to observe for any effect of the injection procedure. Although this treatment route will affect mGlu5 in extrahippocampal structures, previous studies have confirmed an equivalent inhibition of hippocampal LTP occurs with this antagonist dose, as that which occurs following intracerebral injection of MPEP (Naie and Manahan-Vaughan, 2004; Tsanov and Manahan-Vaughan, 2009).
Single-Unit Recordings
In the screening phase, rats were examined once or twice daily for unit activity in a screening box that was visually distinct from, and in a different room to, the test arena. Neural activities were passed through AC-coupled, unity-gain operational amplifiers, which were mounted on a headstage (Axona, UK) connected close to the rat’s head through a socket that fitted onto the microdrive plug. The headstage was linked to a pre-amplifier via lightweight hearing-aid wires. The buffered signal from the headstage was amplified 6,000–30,000 times in the pre-amplifier and then digitized (48 kHz) and bandpass filtered (0.6–7 kHz) in the dacqUSB system unit (Axona, UK). Each tetrode could be recorded differentially being referenced by one electrode of another tetrode. One of the recording channels was dedicated to EEG recording. The position of the rat was monitored by a video camera mounted directly above the platform and converted into x–y coordinates by a tracking system which detected a small light mounted on the headstage near the rat’s head.
Data Analysis
Data analysis was performed using the Tint analysis software (Axona, UK). The collected waveforms from the system unit were displayed as clusters by plotting the peak-to-peak amplitude of each spike on one electrode against that on each of the other three. The clusters were isolated initially by hand. Complex spike cells with one or two firing fields were separated on the basis of spike shape, firing rate, and firing location. At least 50 spikes were isolated for each cluster. Once the cluster was isolated, a boundary of cluster was automatically generated by Tint software (Axona, UK). Then the same boundary was applied for cluster cutting in all other trials. After the cluster cutting, firing rate maps for each cell were visualized and smoothed using Tint, which divided the camera view arena into 64 × 64 square bins with a side length of 2.5 cm. The firing rate for a given cell in each bin indicated the spike number divided by dwell time in that bin. The firing rate maps were presented in color with a lowest firing rate (i.e., 0 Hz) in blue and the highest in red. Place field was defined as the contiguous group of pixels possessing a firing rate higher than half of the peak firing rate and covering <60% of the size of the recording arena. If a place cell was identified with one or more place fields, recordings were repeated two to three time on the same day and at least once more on the second day to verify its stability. If no qualified cell activity was identified, the tetrodes were advanced 25–50 µm and rats were returned to their home cages for at least 2 h. The maximum movement of tetrodes per day was 150 µm.
For each place cell, the firing rate map for each trial was examined to determine: (1) place field size; (2) average firing rate; (3) peak firing rate; (4) mean infield firing rate; (5) mean outfield firing rate; (6) spatial information content; and (7) spatial coherence. The size of the place field was calculated as the percentage of the recording arena by the place field. The average firing rate was determined by dividing the number of spikes that occurred over the entire trial by the duration of the trial. The peak firing rate was determined as the highest firing rate of all pixels within the place field of the cell. Mean infield and outfield firing rates were defined as the mean values for the firing rates of all pixels within (infield) and outside (outfield) the place field. The spatial information content, measured in bits/spike, is a measure of how much information about the spatial location of the animal is contained within the activity of the cell. It was calculated using the methods described by Skaggs et al. (1993). Spatial coherence is a measure of the spatial contiguity of the neuron’s activity. It determines the “smoothness” of place fields in firing ratemaps. It was calculated in steps described by Muller and Kubie (1989).
The similarity between firing ratemaps in each pair of trials was analyzed using a correlation procedure as follows. Each map was decomposed into a 32 × 32-element matrix. Each pixel in one matrix was correlated, by a Pearson’s correlation, with its equivalent pixel in the second map. Pixels with a zero firing rate in both metrics were discarded. Correlation measures were not applied to trial-pairs, in which cells turned on/off due to remapping.
Statistics
Various statistical analyses were applied to measures of place cell characteristics. Normality test (Kolmogorov–Smirnov) was first applied to each data set to examine whether data match the pattern expected if the data was drawn from a population with a normal distribution. T tests were applied to analyze differences between groups when data were pooled from all trials in familiar or novel environments. Two-way analysis of variance (ANOVA) with Fisher’s post hoc tests were applied to detect differences between groups across multiple trials. Statistical significance was defined as P ≤ 0.05.
Behavioral Apparatus
All screening for units took place in an open field square box with a floor of dimensions 80 cm × 80 cm, and walls 70 cm high. When well-isolated place cells with stable fields were confirmed, experiments were performed following the experimental protocol described below (Fig. 1). Two boxes were used in this experiment. A square box, which was previously described for screening, served as a familiar environment. Rats spent at least 10 min per day for longer than 2 weeks in this box. A circular box (diameter, 85 cm; height, 90 cm) was used as a novel environment. The color, texture and material of the floor and walls were all different between both.
Figure 1.
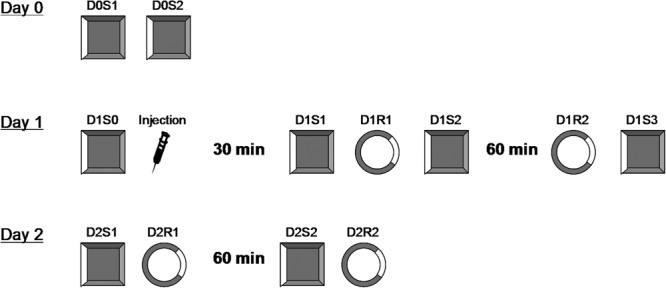
Overview of the experimental protocol for place cell recordings. All trials during 3 experimental days are illustrated. Two boxes were used in this experiment. A square box, which was previously used for unit screening, served as a familiar environment. Rats spent at least 10 min per day for longer than 2 weeks in this box. A circular box was used as a novel environment. The color, texture and material of the floor and walls were all different between both. On Day 0, place cells were identified (D0S1) and confirmed (D0S2) in the square familiar box. On Day 1, cells were confirmed again in the familiar box (D1S0), after which rats were injected i.p. with either vehicle or MPEP. After the injection, rats were kept in their home cages for 30 min before D1S1 started. Trial D1S1 was to test whether pre-established place fields were compromised by the injection. In trial D1R1, rats were placed into a novel circular box. The formation of place fields was observed in this trial. Trial D1S2 is to test whether exposure to a novel environment disrupted place fields established in familiar environment in both groups of animals. These trials took a total time of about 1 h, after which rats were returned to their homecages and remained for 1 h. Trial D1R2 allowed us to know if newly formed place fields in the novel environment were still stable after an interval of 2 h. Stability of place fields in the familiar environment was checked again in D1S3. On day 2, place field stability was first checked in the familiar environment in D1S1. Trial D2R1 allowed us to examine whether newly formed place fields were stable after a long interval of about 24 h. One hour later, trial D2S2 provided yet another check of stability, and trial D2R2 allowed us to see if the place fields in the novel environment were the same as in trial D2R1. The protocol for day 1 and day 2 consisted of 10 trials, each for 5 min, with an inter-trial interval of 10 min, during which the rats were removed from the recording box and returned to their home cages.
Experimental Protocol
The experimental structure is shown in Figure 1. Animals were first allowed to explore the familiar or novel box for 1.5 min before the first trial of a day. This was to minimize the factor that place fields were less stable during the first minutes. In the following descriptions, the code “D” stands for the experimental day. The “S” stands for the “square arena” which is familiar to animals and “R” stands for the “round arena,” which is novel to animals. On Day 0, at least two place cells were identified (D0S1) and confirmed (D0S2) in the square familiar box. On Day 1, cells were confirmed again in the familiar box (D1S0). If their firing pattern on the spike clusters were not shifted from the ones from last trials, rats were injected i.p. with either vehicle or MPEP. If the spike clusters were shifted, experiment stopped and returned to D0S1. After the injection, rats were kept in their home cages for 30 min before D1S1 started. Trial D1S1 was to test whether injection of vehicle/MPEP affected place fields in the familiar environment which were already established. In trial D1R1, rats were placed into a novel circular box. The question for this trial was to ask whether MPEP affected the formation of place fields for the first time in a new environment. Trial D1S2 was used to test whether, after injection with MPEP, exposure to a novel environment disrupted place fields established in a familiar environment, i.e., in D1S1. These trials took a total time of about 1 h, after which rats were returned to their home cages and were kept there for 1 h. Trial D1R2 allowed us to find out if newly formed place fields in the novel environment were still stable after an interval of 2 h, and trial D1S3 provided both a check of cell stability in familiar environment and a baseline for day 2 recordings. On day 2, trial D2S1 allowed us to check again for place field stability in the familiar environment, and trial D2R1 allowed us to examine whether newly formed place fields were stable after a long interval of about 24 h. Trial D2S2 provided yet another check of stability, and trial D2R2 allowed us to see if the place fields in the novel environment were the same as in trial D2R1. The protocol for day 1 and day 2 consisted of 10 trials, each for 5 min, with an inter-trial interval of 10 min, during which the rats were removed from the recording box.
Histological Analysis
The location of the recording and stimulation electrodes was verified by postmortem histological visualization. The tissue was fixed, coronal slices were obtained and Nissl stained (Manahan-Vaughan et al.,1998). Animals with misplaced electrodes were not included in the data analysis.
Results
A total of 52 cells were recorded from the CA1 region of 13 rats (7 rats in the control group and 6 in the MPEP group), of which 27 cells were recorded in the control group and 25 cells in the MPEP group. Firing rate maps in all trials are shown in the Supporting Information figures (Figs. S1 and S2). Three cells (cell 14, 23, 27) in the control group (11%) and three cells (cell 2, 4, 10) in the MPEP group (12%) became silent when animals were placed in the novel environment. One cell in the control group (cell 17) and one cell in the MPEP group (cell 9) were lost on Day 2. They were excluded from the statistical analysis. One cell (cell 20 in the control group) became silent in the familiar environment on Day 2, but retained its firing pattern in the novel environment. Two cells in the MPEP group (cell 17, 25) fired only in the novel environment.
Pre-established Place Fields in a Familiar Environment Were Not Affected by mGlu5 Antagonism
In the familiar environment, the correlation coefficient between D1S0 and D1S1 indicated high similarity in both the control group (mean ± SEM: 0.78 ± 0.03) and the MPEP group (mean ± SEM: 0.82 ± 0.02; Fig. 2). No significant difference between groups was observed through all trials by ANOVA. This suggests that injection of either vehicle or MPEP did not affect the stability of place fields, which were already established in a familiar environment. High correlation values between D1S1 and D1S2 (mean ± SEM: 0.79 ± 0.04 control and 0.81 ± 0.02 MPEP), D1S2 and D1S3 (mean ± SEM: 0.76 ± 0.03 control and 0.85 ± 0.02 MPEP), D1S3 and D2S1 (mean ± SEM: 0.83 ± 0.02 control and 0.81 ± 0.03 MPEP), D2S1 and D2S2 (mean ± SEM: 0.79 ± 0.03 control and 0.84 ± 0.02 MPEP) suggest that the maintenance of a pre-established place field in a familiar environment did not require mGlu5 activation. Examples of three cells from the control group (Fig. 3a) and three cells from the MPEP group (Fig. 4a) show the kind of firing patterns that were recorded in the familiar environment.
Figure 2.
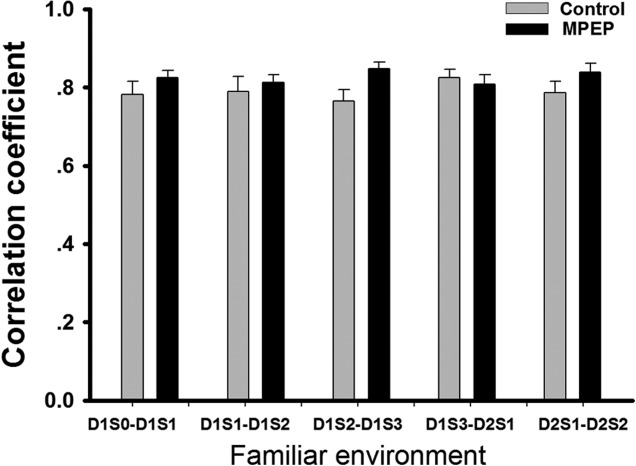
Stable spatial correlations of firing pattern in each pair of trials in the familiar environment. The correlation coefficient was calculated from the trial-pairs indicated, in the familiar environment. ANOVA was applied to detect differences between control and MPEP groups. No significant difference was observed. Bar chart shows mean value ± SEM.
Figure 3.
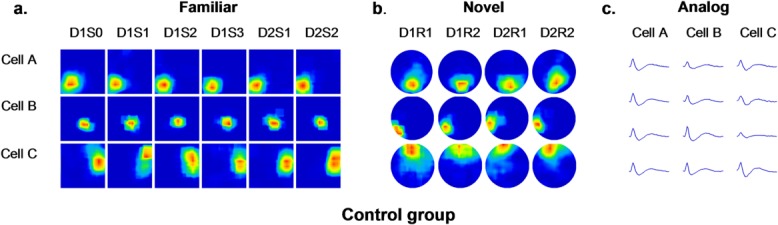
Place cells of control animals showed stable firing patterns in the familiar and novel environment. Firing rate maps of three cells detected through all trials in the familiar environment are shown in (a). Recordings were conducted in a square box. All cells showed stable firing patterns through all trials in the familiar environment. Firing rate maps of three cells through all trials in the novel environment are shown in (b). Recordings were conducted in a circular box. All cells showed stable firing patterns through all trials in the novel environment (c). Spike waveforms of corresponding cells recorded by tetrodes in a time window of 2 msec are shown.
Figure 4.
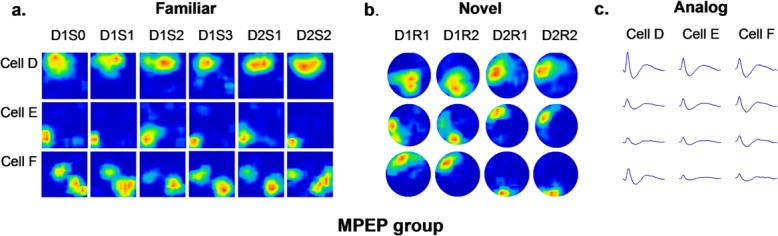
Place cells of MPEP-treated animals exhibited remapping 24 h after exploration in the novel environment. (a) Firing rate maps of three cells through all trials in the familiar environment. Recordings were conducted in a square box. All cells showed stable firing patterns through all trials in the familiar environment (b). Firing rate maps of three cells through all trials in the novel environment. Recordings were conducted in a circular box. Cells showed stable firing patterns in two trials on the first day, but remapped on the second day. The remapping firing patterns were preserved and repeatable after a following recording trial on the second day. (c) Spike waveforms of corresponding cells recorded by tetrodes in a time window of 2 msec are shown.
Formation of Place Fields in Novel Environment Was Not Impaired by mGlu5 Antagonism
As shown in Figure 2, the firing pattern of place cells in the familiar environment showed a high spatial correlation between D1S0 and D1S1 in both groups (mean ± SEM: 0.78 ± 0.03 in control group; 0.82 ± 0.02 in MPEP group. Exposure to the novel environment induced the formation of new spatial firing patterns in both groups of animals (Fig. 5). This was evidenced by a significantly lower spatial correlation between D1S1 and D1R1, which was the first trial where the animals entered the novel environment (mean ± SEM: 0.05 ± 0.05 control and 0.11 ± 0.05 MPEP; indicating remapping) (ANOVA: F(1, 87) = 302.91, P < 0.05). There was no difference in spatial correlations in D1S1-D1R1 between control and MPEP groups.
Figure 5.
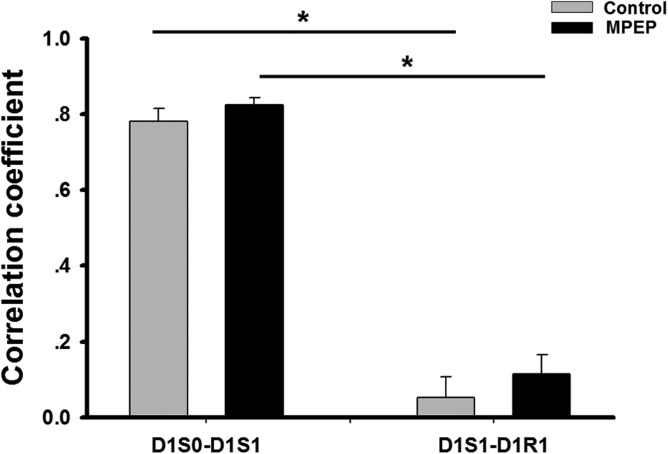
Spatial correlations between familiar-familiar and familiar- novel environments indicated remapping upon entering a novel environment. The correlation coefficients were calculated from indicated trial-pairs in both groups. Correlation contents of D1S1-D1R1 were significantly lower than those of D1S0-D1S1 in both groups. No significance was observed between groups. Bar chart shows mean value ± SEM. (ANOVA + Fisher’s post hoc test; *: P < 0.05).
Long-term Place Field Stability, But Not Short-term Stability, in a Novel Environment is Impaired by Antagonism of mGlu5
In the novel environment, short-term stability of newly formed place fields was unaffected by vehicle or MPEP, which was evidenced by a high correlation between D1R1 and D1R2 (mean ± SEM: 0.65 ± 0.05 control and 0.70 ± 0.06 MPEP; Fig. 6). However, the correlation between D1R2 and D2R1 in the MPEP group (mean ± SEM: 0.18 ± 0.08) was significantly lower than that in the control group (mean ± SEM: 0.66 ± 0.06), suggesting impaired long-term stability (ANOVA + Fisher’s post hoc tests: P < 0.05). This can be seen in the examples of firing patterns of cells in the control group (Fig. 3b) and MPEP group (Fig. 4b), and suggests the global remapping occurred between Day 1 and Day 2 in animals that were treated by MPEP.
Figure 6.
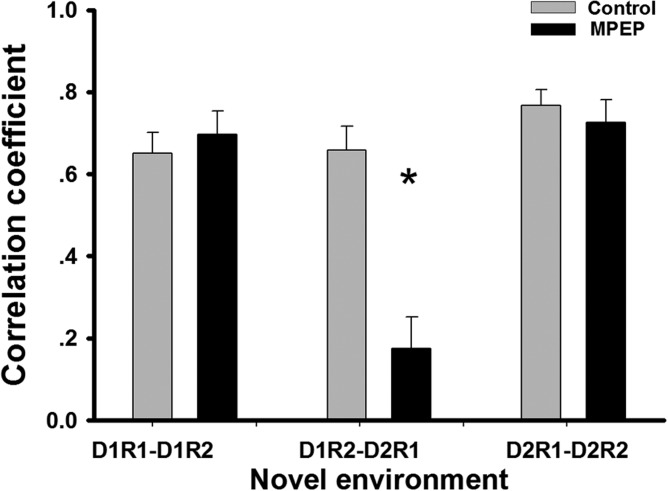
Correlations of firing pattern in each trial-pair in the novel environment showed remapping 24 h after an initial exposure to a novel environment. The correlation coefficients were calculated from indicated trial-pairs in the novel environment. Significantly decreased spatial correlations were observed in the MPEP group in D1R2-D2R1, while those in other trial-pairs in the MPEP group were as high as those in the control group. Bar chart shows mean value ± SEM. (ANOVA + Fisher’s post hoc test; *: P < 0.05).
Spatial Information Content Obtained in a Novel Environment is Impaired by Antagonism of mGlu5
In the familiar environment, spatial information of place cell firing in both control and MPEP groups remained at a similarly high level (Fig. 7a) (ANOVA: F(1, 250) = 0.18572, P > 0.05), whereas, in the novel environment, spatial information content was lower in the MPEP group (Fig. 7a) (ANOVA: F(1, 150) = 14.165, P < 0.05).
Figure 7.
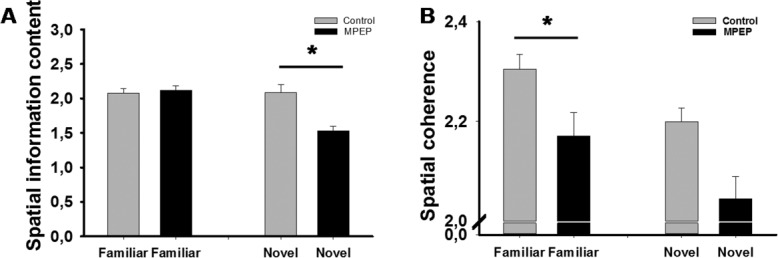
Spatial information contents and spatial coherence were impaired in the MPEP group. Spatial information contents (A) and spatial coherence (B) of place fields in the familiar and novel environment are illustrated. Spatial information in the control and MPEP group was equally high in the familiar environment. But in the novel environment, spatial information was lower in the MPEP group. Spatial coherence in the MPEP group was significantly decreased in the familiar environment but only showed a non-significant decrease in the novel environment (ANOVA, *: P < 0.05). Bar chart shows mean value ± SEM.
Spatial Coherence was Impaired in Both Familiar and Novel Environment by Antagonism of mGlu5
In the familiar environment, the spatial coherence of place cell firing was lower in the MPEP group compared to the control group (Fig. 7b) (ANOVA: F(1, 250) = 15.440, P < 0.05). In the novel environment, the mean value of spatial coherence was also lower in the MPEP group (Fig. 7b). However, no significant difference was observed between the two groups (ANOVA: F(1, 150) = 3.43, P = 0.06).
Peak Firing Rate and Infield Firing Rate are Lower in a Novel Environment When mGlu5 Receptors are Antagonized
In the MPEP group, the peak firing rate and the infield firing rate of the place cells were significantly lower in the novel environment compared to the rates observed in the familiar environment, whereas average firing rates and outfield firing rates remained stable (Table 1, t test: P < 0.001). In the control group, all firing rate measures remained stable in both the familiar and novel environments (Table 1, t test: P < 0.05).
Table 1.
Average Firing Rate, Peak Firing Rate, Infield and Outfield Firing Rate of Cells in Control and MPEP Group Between Familiar and Novel Environment
| Control group | MPEP group | |||||
|---|---|---|---|---|---|---|
| Firing rate (Hz) | Familiar environment | Novel environment | Significance | Familiar environment | Novel environment | Significance |
| Average | 0.28 ± 0.02 | 0.31 ± 0.05 | n.s. | 0.29 ± 0.02 | 0.25 ± 0.03 | n.s. |
| Peak | 3.06 ± 0.20 | 2.81 ± 0.44 | n.s. | 3.69 ± 0.19 | 2.12 ± 0.19 | P < 0.005 |
| Infield | 2.18 ± 0.15 | 1.99 ± 0.32 | n.s. | 2.37 ± 0.13 | 1.39 ± 0.15 | P < 0.05 |
| Outfield | 0.16 ± 0.01 | 0.18 ± 0.03 | n.s. | 0.17 ± 0.01 | 0.16 ± 0.02 | n.s. |
t test; n.s.: non-significant.
Average Velocity and Place Field Size are Unaffected by mGlu5 Antagonism
Average velocity and place field size were normalized to their average values through all trials. No significance was observed between two groups by ANOVA (Fig. 8).
Figure 8.
Average running speed and place field size of place cells in each trial. (a) Running speed of rats was normalized to its average speed throughout all trials. No significant difference was observed between groups. (b) The size of each place field was calculated with regard to the size of the recording box in each trial and was normalized to its average size throughout all trials. No significant difference was observed between groups. Bar chart shows mean value ± SEM (ANOVA, P > 0.05).
Discussion
In this study, we provide the first evidence that the metabotropic glutamate (mGlu) receptor, mGlu5 is important for long-term information storage at the level of place fields. We observed that antagonism of mGlu5 prevents long-term place field stability, reduces information content and reduces place cell firing rates in a novel environment. This suggests that mGlu5 is necessary for the stabilization of spatial information that is encoded by place cells. This observation creates an intriguing link between place cells, hippocampus-dependent spatial memory and synaptic plasticity, as it has been widely documented that mGlu5 is essential for both latter phenomena.
The role of mGlu5 in synaptic plasticity and hippocampus-dependent spatial memory has been extensively studied (see Mukherjee and Manahan-Vaughan, 2013, for review). Activation of the receptor is necessary to enable persistent synaptic plasticity (Naie and Manahan-Vaughan, 2004; Popkirov and Manahan-Vaughan, 2011) whereby the NMDA receptor preferentially enables induction of plasticity to occur. Thus, at least for the CA1 region, the NMDA receptor enables the initial calcium influx required for the immediate change in synaptic strength that occurs followed patterned afferent stimulation (Dudek and Bear, 1992; Mulkey and Malenka, 1992; Manahan-Vaughan, 1997) and which enables plasticity to endure for several minutes, whereas activation of mGlu5 strengthens the synaptic response such that it endures for at least 4 h (Naie and Manahan-Vaughan, 2004; Popkirov and Manahan-Vaughan, 2011). To enable plasticity that endures for longer periods of several hours, days or more, protein synthesis must be triggered (Frey et al., 1988; Manahan-Vaughan et al., 2000).
MGlu5 mediates the prolongation of synaptic plasticity through a variety of mechanisms. As a group I mGlu receptor subtype, it is positively coupled to phospholipase C (Nakanishi et al., 1994; Pin and Duvoisin, 1995) and when activated, enables that calcium is released from intracellular stores. The receptor can also facilitate calcium entry through NMDA receptors (Mannaioni et al., 2001) and activates somatic calcium transients, thus affecting frequency accommodation in hippocampal synapses (Niswender and Conn, 2010). MGlu5 also enables neuronal oscillations in the hippocampus that are presumably the vehicle for neuronal information transfer across this structure (Bikbaev et al., 2008), and is intrinsically involved in the induction and maintenance phases of both persistent LTP (Balschun and Wetzel, 2002; Naie and Manahan-Vaughan, 2004; Manahan-Vaughan and Braunewell, 2005; Neyman and Manahan-Vaughan, 2008) and LTD (Neyman and Manahan-Vaughan, 2008). Prolonged mGluR5 blockade leads to a decrease of both theta and gamma power in the dentate gyrus (Bikbaev et al., 2008) and activation of mGlu5 increases excitability in layer V pyramidal neurons (Sourdet et al., 2003). Both findings suggest that mGlu5 might be essential in the precise temporal encoding required for spatial memory. Changes in neuronal oscillations mediated by mGlu5 antagonism could thus have affected place cell stability by reducing available information content. In line with this, mGlu5 is essential for many forms of hippocampus-dependent learning including inhibitory avoidance learning (Simonyi et al., 2007), spatial alternation (Riedel et al., 1999; Balschun et al., 2002), spatial context (object-place) learning (Popkirov and Manahan-Vaughan, 2011), spatial working and reference memory (Naie and Manahan-Vaughan, 2004), object recognition memory (Barker et al., 2006), spatial learning in a water maze (Richter-Levin et al., 1994; Bordi et al., 1996). Alterations in mGlu5 function and/or expression are also a major factor in fragile-X mental retardation (Giuffrida et al., 2005; Fatemi et al., 2011).
In light of the substantial body of evidence supporting an intrinsic role for mGlu5 in hippocampus-dependent learning and synaptic plasticity, it is perhaps not all that surprising that we identified a role for mGlu5 in place cell encoding. A not too delicate interplay between place cell encoding and LTP has been described, whereby afferent stimulation to the hippocampus to induce LTP obliterates place fields and facilitate remapping (Dragoi et al., 2003) The exact nature of the relationship between LTP and place cell encoding is unclear, but it may be that LTP serves under certain circumstances to generate a tabula rasa after which new information may be stored in the hippocampus, or that it identifies and selects synapses that engage in information encoding of a particular (spatial) experience (Kemp and Manahan-Vaughan, 2007). Nonetheless, it is clear that both LTP and LTD play an important role in the enablement of long-term information storage by the hippocampus (Kemp and Manahan-Vaughan, 2007,2008) and that both plasticity phenomena require mGlu5 activation (Mukherjee and Manahan-Vaughan, 2013).
In this study, the main characteristics of place cells in the familiar environment, such as spatial correlation, spatial information content and place field size, were not affected by mGlu5 antagonism. This is consistent with reports on the effects of blocking NMDA receptors (Kentros et al., 1998), blocking protein synthesis in the brain (Agnihotri et al., 2004) or deleting the transcription factor zif268/egr1 (Renaudineau et al., 2009) on place fields. Thus, once the spatial representation had been created, mGlu5 receptors were not required. However, upon entering a novel environment, both vehicle and MPEP-injected rats readily demonstrated remapping. The degree of remapping was highly similar in both groups of animals. Our findings are distinct to a study that explored the contribution of NMDA receptors to place field stability. Here, it was reported the initial remapping process that occurs in animals injected with an antagonist of NMDA receptors, was not complete, whereby the initial firing pattern in the novel environment partially resembled the pattern observed in the familiar environment (Kentros et al., 1998). We can think of two possible reasons for the difference in our findings with regard to mGlu5 antagonism and this study that addressed the effects if NMDA receptor antagonism: the first one is that NMDA receptors, but not the mGlu5 receptors, are partially involved in the initial remapping. NMDA receptors, mGlu5 receptors and protein synthesis are essential for different and contiguous phases of persistent long-term synaptic plasticity. Exposure to a new spatial environment facilitates the expression of persistent synaptic plasticity (Kemp and Manahan-Vaughan, 2004). This phenomenon, referred to as learning-facilitated plasticity is also prevented by NMDA receptor and mGlu5 receptor antagonism (Popkirov and Manahan, 2011), whereby NMDA receptor antagonism prevents the induction of plasticity and mGlu5 antagonism prevents the transition of the early, induction phase of plasticity into a more lasting form. This suggests that the different effects on initial remapping seen during NMDA receptor and mGlu5 receptor antagonism reflect the triggering and contribution of distinct components that parallel the temporal dynamics of long-term synaptic plasticity phenomena. The second possible reason may lie within the saliency of the novel environment. In the NMDA receptor antagonism experiment described above (Kentros et al., 1998), the difference between the novel and familiar environment derived mainly from the color of the cylinder and the color of the cue card. In our study, the shape of the environment became physically changed from a familiar square to a novel circular arena. Thus, is also possible that the difference between the two environments in our study drove complete remapping in both the control and MPEP groups of animals.
In contrast to the lack of necessity for mGlu5 activation for the generation of new place fields, one important finding of this study is that the long-term (>24 h) stability of newly formed place fields in a novel environment was impaired by mGlu5-antagonism (although short-term stability remained intact). This finding offers an interesting parallel to the key role of mGlu5 in enabling long-term plasticity (Mukherjee and Manahan-Vaughan, 2013). Most of the cells in MPEP-injected rats, which initially established place fields in a novel environment, remapped on the second experiment day, when re-exposed to the same environment. This contrasts to the stable firing pattern of place cells in vehicle-injected rats. This finding suggests that mGlu5 is essential for long-term but not short-term stability of spatial representation in the hippocampus. Moreover, in contrast to the stable place cell firing pattern observed in the familiar environment, where spatial memory had been effectively consolidated, place cells of MPEP-treated rats in the non-consolidated novel environment expressed different firing patterns after a long interval of 24 h. This finding suggests that mGlu5 is critical for the stabilization of newly formed spatial representations that have not been consolidated.
Inhibition of mGlu5 might impair the spatial accuracy of place cell firing patterns: place cells in MPEP-injected rats fired in a more sparse and less spatially selective manner compared to those in vehicle-injected rats, as indicated by several place field measures, such as spatial information content and sparsity. This finding contrasts with the reports from others with regard to the lack of involvement of NMDA receptors or protein synthesis in these aspects of place cell encoding. A significant decrease in firing rate measures such as peak and infield firing rates were detected, when the animals moved from the familiar to the novel environment in the MPEP group. One possible explanation is that such changes might be correlated to elevated neuronal excitability. Activation of mGlu leads to enhanced neuronal and an increase in postsynaptic intracellular calcium (Abdul-Ghani et al., 1996). In addition, mGlu5 activates somatic calcium transients (Niswender and Conn, 2010). MGlu5 also changes calcium flow through NMDA receptors (Jia et al., 1998; Mannaioni et al., 2001; Attucci et al., 2001). We observed that antagonism of mGlu5 results in a reduction of spatial information in the novel environment, and a reduction in spatial coherence in both the familiar and novel environments. However, since the mGlu5 antagonist was applied systemically, we cannot exclude the possibility that extrahippocampal effects of the antagonist also influenced on place cell activity.
NMDA receptors and mGlu5 clearly play a very important role in hippocampal synaptic plasticity and information encoding. Antagonism of NMDA receptors not only prevents the induction of many forms of hippocampal synaptic plasticity (Nicoll and Malenka, 1999), it also disrupts experience-dependent place cell expansion (Ekstrom et al., 2001). MGlu5, on the other hand is required for persistent synaptic plasticity, hippocampal neuronal oscillations and long-term spatial learning (Mukherjee and Manahan-Vaughan, 2013). However, synaptic plasticity and place cell stability may require other key neurotransmitter receptors. A key role in persistent synaptic plasticity and learning has also been described, for example, for mGlu1 (Mukherjee and Manahan-Vaughan, 2013) and for group II mGlu receptors (Manahan-Vaughan, 1997; Altinbilek and Manahan-Vaughan, 2009).
In conclusion, our data support that mGlu5 plays an important role in the enablement of the stability and longevity of the encoding of spatial representations by place cells. This, on the one hand emphasises the importance of these receptors for hippocampal functioning, and on the other hand, draws an interesting link between other information storage processes in the hippocampus such as synaptic plasticity and place cell encoding. Taken together with findings of other reports as to the significance of plasticity-relevant factors such as the NMDA receptors and protein synthesis for place cell responses, this suggests that synaptic plasticity and place cell encoding may be highly intertwined.
Acknowledgments
The authors thank Jens Klausunitzer and Juliane Böge for technical assistance and Nadine Kollosch for animal care.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- Abdul-Ghani MA, Valiante TA, Carlen PL, Pennefather PS. Metabotropic glutamate receptors coupled to IP3 production mediate inhibition of IAHP in rat dentate granule neurons. J Neurophysiol. 1996;76:2691–2700. doi: 10.1152/jn.1996.76.4.2691. [DOI] [PubMed] [Google Scholar]
- Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. The long-term stability of new hippocampal place fields requires new protein synthesis. Proc Natl Acad Sci USA. 2004;101:3656–3661. doi: 10.1073/pnas.0400385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinbilek B, Manahan-Vaughan D. Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory. Eur J Neurosci. 2007;26:1166–1172. doi: 10.1111/j.1460-9568.2007.05742.x. [DOI] [PubMed] [Google Scholar]
- Altinbilek B, Manahan-Vaughan D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience. 2009;158:149–158. doi: 10.1016/j.neuroscience.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Attucci S, Carla V, Mannaioni G, Moroni F. Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. Br J Pharmacol. 2001;132:799–806. doi: 10.1038/sj.bjp.0703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of group I metabotropic glutamate receptors blocks spatial learning in rats. Neurosci Lett. 1998;249:41–44. doi: 10.1016/s0304-3940(98)00388-7. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Balschun D, Manahan-Vaughan D, Wagner T, Behnisch T, Reymann KG, Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learn Mem. 1999;6:138–152. [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006;13:178–186. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn PJ, Nicoletti F, Manahan-Vaughan D. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS One. 2008;3:e2155. doi: 10.1371/journal.pone.0002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, Marcon C, Chiamulera C, Reggiani A. Effects of the metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology. 1996;35:1557–1565. doi: 10.1016/s0028-3908(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsaki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields.”. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2011;60:1221–1226. doi: 10.1016/j.neuropharm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Czurko A, Csicsvari J, Buzsaki G. Firing rate and theta-phase coding by hippocampal pyramidal neurons during ’space clamping’. Eur J Neurosci. 1999;11:4373–4380. doi: 10.1046/j.1460-9568.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Sacktor TC. Long-term potentiation and long-term depression are induced through pharmacologically distinct NMDA receptors. Neurosci Lett. 1997;226:107–110. doi: 10.1016/s0304-3940(97)00252-8. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci. 2000;20:RC81. doi: 10.1523/JNEUROSCI.20-12-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: Master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Klausnitzer J, Kulla A, Manahan-Vaughan D. Role of the group III metabotropic glutamate receptor in LTP, depotentiation and LTD in dentate gyrus of freely moving rats. Neuropharmacology. 2004;46:160–170. doi: 10.1016/j.neuropharm.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group III metabotropic glutamate receptors modulate long-term depression in the hippocampal CA1 region of two rat strains in vivo. Neuropharmacology. 2000;39:1952–1958. doi: 10.1016/s0028-3908(00)00016-2. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Behnisch G, Vieweg S, Reymann KG, Behnisch T. Semi-automated analysis of NMDA-mediated toxicity in digitised colour images from rat hippocampus. J Neurosci Methods. 1998;82:85–95. doi: 10.1016/s0165-0270(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Ngomba RT, Storto M, Kulla A, Catania MV, Chiechio S, Rampello L, Passarelli F, Capece A, Reymann KG, Nicoletti F. An increased expression of the mGlu5 receptor protein following LTP induction at the perforant path-dentate gyrus synapse in freely moving rats. Neuropharmacology. 2003;44:17–25. doi: 10.1016/s0028-3908(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O’Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Manahan-Vaughan D. Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology. 2013;66:65–81. doi: 10.1016/j.neuropharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: Relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-d-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci. 2005;21:411–421. doi: 10.1111/j.1460-9568.2005.03864.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Masu M, Bessho Y, Nakajima Y, Hayashi Y, Shigemoto R. Molecular diversity of glutamate receptors and their physiological functions. EXS. 1994;71:71–80. doi: 10.1007/978-3-0348-7330-7_8. [DOI] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Popkirov SG, Manahan-Vaughan D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb Cortex. 2011;21:501–509. doi: 10.1093/cercor/bhq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschel B, Manahan-Vaughan D. Group II mGluR-induced long-term depression in the dentate gyrus in vivo is NMDA receptor-independent and does not require protein synthesis. Neuropharmacology 49 (Suppl. 2005;1):1–12. doi: 10.1016/j.neuropharm.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudineau S, Poucet B, Laroche S, Davis S, Save E. Impaired long-term stability of CA1 place cell representation in mice lacking the transcription factor zif268/egr1. Proc Natl Acad Sci USA. 2009;106:11771–11775. doi: 10.1073/pnas.0900484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Errington ML, Maegawa H, Bliss TV. Activation of metabotropic glutamate receptors is necessary for long-term potentiation in the dentate gyrus and for spatial learning. Neuropharmacology. 1994;33:853–857. doi: 10.1016/0028-3908(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Riedel G, Casabona G, Platt B, Macphail EM, Nicoletti F. Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacology. 2000;39:1943–1951. doi: 10.1016/s0028-3908(00)00037-x. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Shelat PB, Dopheide MM, Coulibaly AP, Schachtman TR. Differential roles of hippocampal metabotropic glutamate receptors 1 and 5 in inhibitory avoidance learning. Neurobiol Learn Mem. 2007;88:305–311. doi: 10.1016/j.nlm.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs ME, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. Adv Neural Inform Process Syst. 1993;5:1030–1037. [Google Scholar]
- Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509:299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Manahan-Vaughan D. Synaptic plasticity in the adult visual cortex is regulated by metabotropic glutamate receptor, mGluR5. Exp Brain Res. 2009;199:391–399. doi: 10.1007/s00221-009-1965-4. [DOI] [PubMed] [Google Scholar]
- Wiener SI, Paul CA, Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. J Neurosci. 1989;9:2737–2763. doi: 10.1523/JNEUROSCI.09-08-02737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


